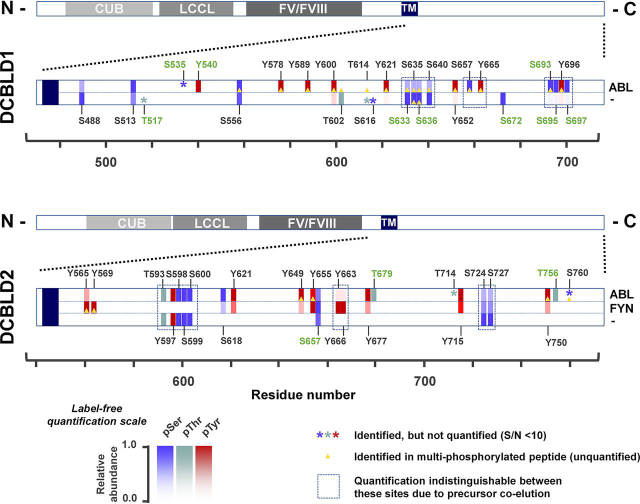
Fig. 1.
Differential phosphorylation of DCBLD family members by FYN and ABL. DCBLD1-FLAG and DCBLD2-FLAG were transiently expressed in 293 cells in the presence and absence of ABL or FYN. Immunoprecipitates (α-FLAG) from 293 lysates were analyzed by SDS-PAGE and Coomassie staining. Bands containing DCBLD1 and DCBLD2 were excised and subjected to proteolytic digest with Trypsin and GluC before analysis via LC–MS/MS. RAW spectra were searched against DCBLD1 or DCBLD2 sequences. Intensities of quantifiable singly phosphorylated peptides (S/N <10) were normalized to unmodified loading control peptides for comparison of relative phosphopeptide intensities across conditions. Relative phosphopeptides abundance is plotted as a spatially resolved heat map across DCBLD1 and DCBLD2 sequences, with blue, green, and red gradients displaying phosphoserine (pSer), phosphothreonine (pThr), and phosphotyrosine (pTyr) abundances, respectively. Unquantifiable phosphorylation sites are indicated by asterisks (*) of the same colors. Sites identified in multiply phosphorylated peptides in each condition are indicated with yellow triangles and tabulated in supplemental Table S1 (supplemental Spectra), but not quantified. In cases where peptides containing multiple potential phosphorylation sites co-eluted and distinct fragment ions demonstrating phosphorylation site localization were not identified, proximal sites were quantified together, indicated by dashed boxes. Notated residues of phosphorylation sites identified in this study that have not yet been curated in the PhosphoSitePlus (phosphosite.org) database are highlighted in green.