Abstract
Purpose:
Women in the Appalachian region have a high mortality rate attributable to cancer in spite of lower incidence of cancer compared with the general US population. Empirical evidence suggests that social support influences cancer outcomes, including adherence to screening guidelines and treatment recommendations. The purpose of this study is to examine the impact of social support on breast cancer screening patterns in a sample of rural Appalachian women.
Methods:
This paper reports the results of analyses of baseline cross-sectional data on breast cancer screening collected during a community-based group-randomized trial. We used the 2010 National Health Institute Survey questionnaires and the Medical Outcomes Study Social Support Survey to assess screening behavior and perceived social support, respectively. Data were analyzed using ANCOVA and ANOVA to assess the mean social support on breast cancer screening patterns (frequently, irregularly, and rarely/never) and relevant sociodemographic variables.
Findings:
Of the eligible participant records analyzed (N = 289), 50% were married, 36% were employed, 20% attended college, 40% had no mammogram in 6 years, and 20% never had mammograms. Overall social support score was high at 73.1 (SD = 18.2). Association between breast cancer screening patterns and social support scores was not statistically significant at α < 0.05 (P value = 0.09).
Conclusions:
Although social support as it measured in this study does not show significant associations with screening patterns, it is important to understand how social network structures may influence screening patterns. Familial and social roles/responsibilities that result in reported social support may also be the barrier to cancer screening and other prevention health behaviors.
Keywords: breast cancer, community based, mammography, oncology, rural Appalachia
1 |. INTRODUCTION
According to the American Cancer Society (ACS), women aged 45 and older should have annual screenings for breast cancer.1 Repeated use of breast cancer screening is crucial for early detection and decreased mortality. Having less than three screenings in a 6-year period increases the risk of late stage diagnosis,2,3 and women who have had no screenings in the past 6 years have the highest risk of have late-stage breast cancer and increased risk for disease-related mortality.3 Breast cancer, the second leading cause of cancer death for women, is a major concern for the Appalachian population.4 Although the incidence rates of breast cancer from 2011 to 2015 are lower among rural Appalachian dwellers (116.5 per 100 000) compared to non-Appalachian women in Kentucky (128.3 per 100 000), mortality rates are higher, 20.7% and 23.9%, respectively.5
The determinants of this disparity in mortality are varied and include individual, community, and environmental considerations. The literature indicates that women who adhere to the ACS guidelines–annual mammograms starting at age 45–may experience a reduction in mortality by as much as 20% to 35% in women aged 50 to 69 years and 20% in women aged 40 to 49 years.6,7 However, women in rural Appalachia are screened at lower rates (68.8%) than women8 in non-Appalachian Kentucky (76.5%) and the nation in general.9 Additional community and environmental determinants that contribute to health disparities in Appalachia include geographic isolation, transportation issues, health care coverage, poverty and unemployment, and an inadequate supply of health care providers.10,11 The culmination of these determinants contribute to low cancer screening rates for rural Appalachian women12 that are well below the Healthy People 2020 goal of 81%.
Moreover, a growing body of evidence suggests that social support influences cancer-related outcomes, including adherence to screening guidelines and treatment recommendations.13,14 Social support, characterized as an aspect of human social relationship by which emotional, instrumental, or financial help can be obtained from the social network that an individual belongs, is a social environment factor that can positively influence preventive health and chronic disease self-management behaviors.15 Moreover, Heaney and Israel16 has conceptualized the relationship between social network and social support that indicates their impact preventive health practices in cancer patients (see Figure 1). Based on this conceptual framework, it is thought that social support can be a key social environment factor in rural Appalachian that can be used to leverage health promotion to mitigate health disparities.
FIGURE 1.
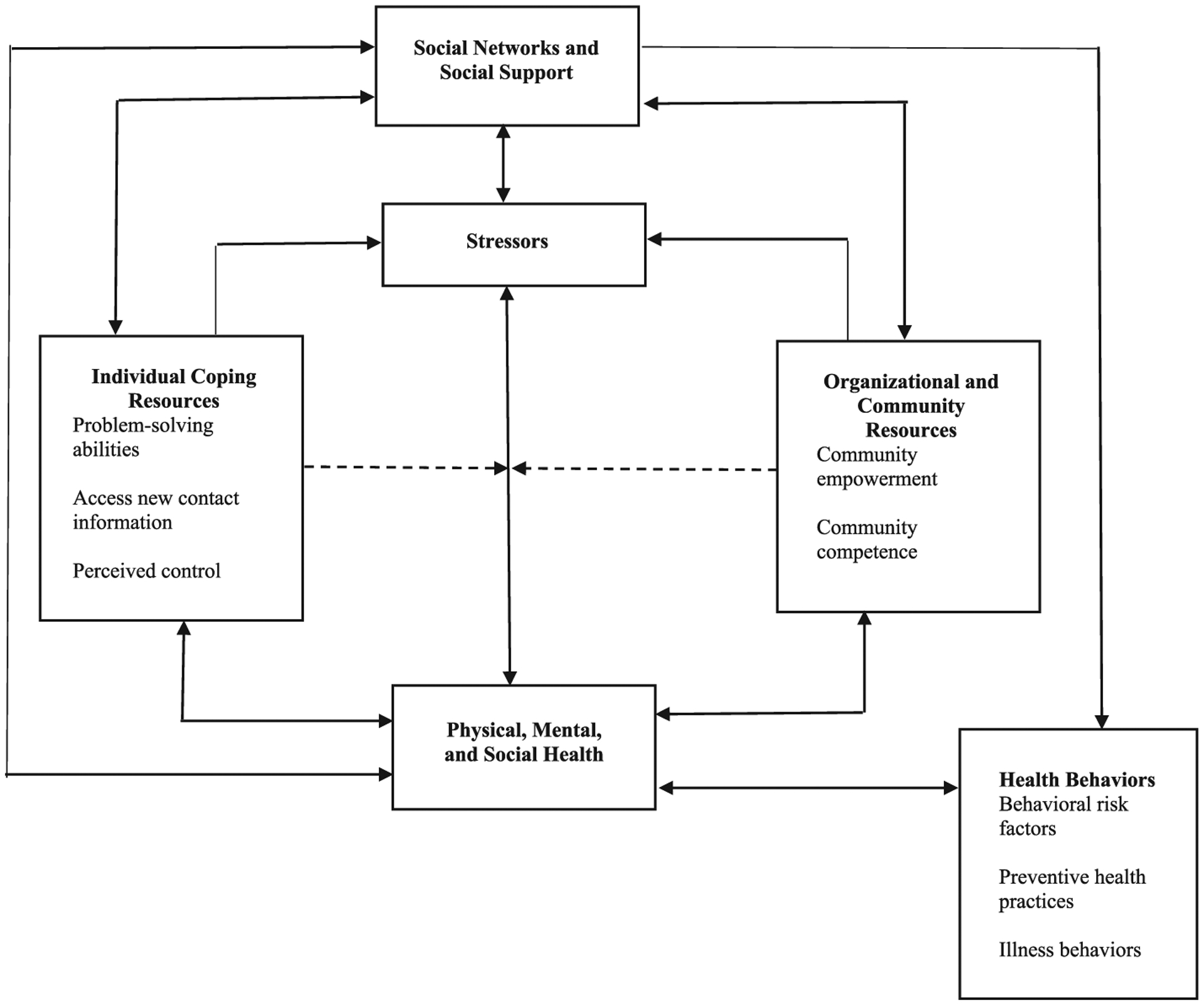
Conceptual model for the relationship of social networks and social support to health
There are several mechanisms through which social support might influence adherence to breast cancer screening recommendations. The role of social support is based on the assumption that the social network, which is those providing social support, is at least partly responsible for determining individual attitudes and behaviors through access to resources and opportunities, and stimuli to perform certain behaviors.17,18 Assistance and support from friends and family may promote screening adherence by encouraging optimism, confidence/self-efficacy, and self-esteem, which are important psychosocial constructs that have been linked to greater screening adherence.19 The practical or tangible support offered by persons in a social network may be instrumental in overcoming barriers to mammography such as child care, transportation, and other physical impediments to screening adherence. Formal support, in the form of provider recommendation, is another possible pathway for social support to influence screening decisions. When physicians or providers are perceived as part of the social support system, the likelihood that women obtain mammograms has been shown to increase.20,21
Furthermore, there is evidence that health-related choices are influenced by the perceived or real attitudes of the people within an environment.22 If those attitudes are negative, they may discourage health-seeking behavior. In addition, perceptions of risk may be influenced by members of the social network; low-risk perception may also have a negative effects. Given the strong family ties and community relationships that may be present for persons in rural Appalachia and the devastating breast cancer mortality that is pervasive, understanding the relationship between social support and breast cancer screening behaviors may provide important insights into designing appropriate interventions to promote and facilitate increased breast cancer screening. Therefore, the purpose of this paper is to examine the relationship between social support and breast cancer screening patterns, using ACS guidelines, in a sample of rural Appalachia Kentucky women.
2 |. METHODS
This paper reports the results of a subanalysis of baseline cross-sectional data on breast cancer screening collected during a community-based delayed intervention, group-randomized trial, “Faith Moves Mountains: A Community Based Participatory Research Appalachian Wellness & Cancer Prevention Program” (R24MD002757: Schoenberg) in rural Appalachia. Faith Moves Mountains was designed in part to increase cancer screenings among rural Appalachian women who do not receive cancer screenings according to recommended guidelines. The study was conducted in four rural counties in rural Appalachia. Of the counties included in this study, the two larger counties were composed of about 30 000 residents and had a Rural-Urban Continuum Code of 7 (“urban population of 2,500 to 19,999, not adjacent to a metro area”). The two smaller counties have 17 649 and 25 277 residents and a Rural-Urban Continuum Code of 9 (“completely rural or less than 2,500 urban population, not adjacent to a metro area”).23
2.1 |. Recruitment
2.1.1 |. Faith settings
The project was conducted in partnership with multidenominational (Pentecostal, Catholic, Baptist, etc) churches in six rural Appalachia Kentucky counties. This is the second in a series of projects conducted in this area, allowing for recruitment to be based largely on expansion of an existing network of previously established relationships. Recruitment details for the initial project are reported elsewhere.24 Project recruitment involved initial contact with church leaders by Faith Moves Mountains project staff to describe the project, followed by a series of meetings to establish trust and lay the groundwork for the project, including kick-off events where church leadership was invited to a presentation regarding the project. We identified church liaisons to facilitate communication with church leaders and potential participants, including arranging information sessions during a lunch. Thirty-two faith-based institutions (referred to throughout as “churches”) in the four rural Appalachian Kentucky counties are included in this study. These congregations were diverse in their sizes, denominations, and membership.
2.1.2 |. Participants
Eligibility included (1) English speaking; (2) ≥46 years; (3) having no personal history of breast cancer; and (4) not having had a mammogram within the past 12 months. We based our eligibility criteria on the recommendations of the ACS, which indicates that women aged 45 years and older should receive a mammogram annually.1 Eligible and interested individuals were enrolled. Written informed consent was obtained at that time, and contact information was gathered. Participants were contacted within 2 weeks, and the baseline interview was conducted by project staff at the participant’s home or other convenient location. All procedures and protocols were approved by the institutional review board at the University of Kentucky (IRB# 08–0119-P2H). This paper reports on baseline data from 289 women enrolled in the breast cancer screening module who were recruited from 32 churches.
2.2 |. Measures
For this analysis, we included variables that have been previously shown to influence adherence to breast screening, such as age, marital status, educational status, income and current financial status, health insurance status, and employment status. These variables were chosen because of their demonstrated relationship with cancer screening.25 To ascertain consistency with breast cancer screening recommendations, we used the 2010 NHIS questionnaires. Participants were asked about their most recent date for mammography/clinical breast exam/breast MRI in the last 6 years. We did not include women who could not have had at least a 6-year history of mammography use, per the ACS guidelines; therefore, only women ≥46 years were included. Responses for the number of mammograms in the last 6 years were classified into three categories: 1, none; 2, one or two; and 3, three or more, allowing us to gain insights into screening patterns since repeated screening is critical for early detection and decreased mortality. While all participants were out of compliance with current screening guidelines, the participants who had been screened three or more times in the past 6 years were considered frequently screened, the participants who had one or two mammograms in the past 6 years irregularly screened, and the ones who had no screenings in the past 6 years rarely screened. The participants were grouped this way because repeated use of breast cancer screening is crucial for early detection and decreased mortality.
Social support was measured using the Medical Outcomes Study (MOS) Social Support Survey.26 The MOS consists of four separate social support subscales: (1) emotional or informational, (2) tangible, (3) affectionate, and (4) positive social interactions, and an overall functional social support index. A higher score for an individual scale or for the overall support index indicates more support. The validity and reliability of the MOS Social Support Survey Instrument has been established,26 and the instrument has been used previously with rural residents.27,28 The reliability of the scale in this sample was strong (α = 0.97).
2.3 |. Analysis
Data analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, North Carolina). Descriptive statistics were obtained to describe the sample characteristics, including breast cancer screening frequency and mean social support scores. Chi-square analyses were conducted to identify significant associations between selected demographic variables and breast cancer screening. ANOVA was used to examine the relationships between age, current financial status, income, education, marital status and education, and mean social support score as well as the MOS subscales. Finally, the association between social support and breast cancer screening patterns was examined using ANCOVA adjusting for age, race, marital status, education, current financial status, income, health insurance, and employment status.
3 |. RESULTS
3.1 |. Sample characteristics
Three hundred and forty-five women were screened for the breast cancer module. Of those, 322 had not been diagnosed with breast cancer, and 295 had not had a mammogram in the last 12 months. Two individuals who were screened declined to enroll in the study, and an additional four participants dropped out, resulting in a sample size of 289 (see Table 1). Consistent with the racial and ethnic demographics29 of Appalachian Kentucky, 97% of participants were white and 3% were black. Age was dichotomized into 46 to 55 years (47%) and ≥55 years (53%) due to the age distribution. Most samples had not obtained a high school diploma (62%), were married (52%), reported being employed (43%), and perceived their incomes to be inadequate or barely adequate with most reporting sometimes struggling financially (54%). Of note, over one-third (38%) described having just enough income to get by. Consistent with this finding, more than half the sample reported an income of less than $25 000 per year (70%), and almost one-third of the sample (29%) reported having no form of health insurance. Regarding breast cancer screening history, 48% of the sample reported having never received a mammogram, approximately 32% had been screened only once or twice in the past 6 years, and 20% were regularly screened.
TABLE 1.
Demographic characteristics (N = 289)
| Breast Cancer Screening Patterns | |||||
|---|---|---|---|---|---|
| Variable | N (%) | Rarely Screened (%) | Irregularly Screened (%) | Regularly Screened (%) | T Statistic (df), P value |
| Age | X2(2) = 1.3, P = 0.525 | ||||
| 46 to 54 years | 136 (47) | 68 (49) | 45 (48) | 23 (40) | |
| 55 years and older | 153 (53) | 71 (51) | 48 (52) | 34 (60) | |
| Race | X2(2) = 0.4, P = 0.827 | ||||
| White | 281 (97) | 136 (98) | 90 (97) | 55 (96) | |
| Black | 8 (3) | 3 (2) | 3 (3) | 2 (4) | |
| Marital status | X2(4) = 4.0, P = 0.399 | ||||
| Married/partnered | 150 (52) | 65 (47) | 50 (54) | 35 (61) | |
| Separated/separated/widowed | 128 (44) | 68 (49) | 39 (42) | 21 (37) | |
| Never married | 11 (4) | 6 (4) | 4 (4) | 1 (2) | |
| Education | X2(4) = 5.3, P = 0.260 | ||||
| Less than high school | 178 (62) | 94 (68) | 53 (57) | 31 (54) | |
| High school diploma/general education diploma | 46 (16) | 21 (15) | 16 (17) | 9 (16) | |
| Some college | 65 (22) | 24 (17) | 24 (26) | 17 (30) | |
| Current financial status | X2(4) = 11.9, P = 0.018 | ||||
| Just about enough | 111 (38) | 45 (32) | 34 (37) | 32 (56) | |
| Sometimes struggle | 155 (54) | 85 (61) | 49 (53) | 21 (37) | |
| More than needed | 23 (8) | 9 (6) | 10 (11) | 4 (7) | |
| Annual household income | X2(4) = 4.6, P = 0.331 | ||||
| ≤$25 000 | 201 (70) | 105 (76) | 60 (65) | 36 (63) | |
| $25 001 to $35 000 | 33 (11) | 13 (9) | 12 (13) | 8 (14) | |
| $35 001 and up | 55 (19) | 21 (15) | 21 (23) | 13 (23) | |
| Health insurance | X2(4) = 2.5, P = 0.645 | ||||
| Private/employer provider | 94 (33) | 43 (31) | 29 (31) | 22 (39) | |
| Government insurance | 111 (38) | 56 (40) | 33 (35) | 22 (39) | |
| Uninsured | 84 (29) | 40 (29) | 31 (33) | 13 (23) | |
| Employment status | X2(2) = 2.2, P = 0.341 | ||||
| Employed | 125 (43) | 56 (40) | 46 (49) | 23 (40) | |
| Unemployed | 164 (57) | 83 (60) | 47 (51) | 34 (60) | |
3.2 |. Social support, breast cancer screening, and demographic characteristics
Overall social support was moderately high with an average total score of 73.1 (range, 27–95; SD = 18.2). Yet age and educational level were not associated with social support. However, marital status (never married, M = 80.3, F = 10.8, P < 0.001); greater financial security (M = 87.1, F = 14.7, P < 0.001); household income > $35 000 (M = 81.7, F = 8.0, P < 0.001); and private insurance (M = 76.8, F = 3.33, P < 0.037) were associated with higher overall social support. The relationship between social support subscales and demographic variables was reflective of their relationship with the overall social support score. Higher scores on the social support subscales were associated with the following: emotional/informational support with never been married (M = 32.7, F = 4.2, P = 0.016); greater financial security (M = 36.6, F = 11.1, P < 0.001); household income of >$35 000 (M = 34.0, F = 6.3, P = 0.002); and private insurance (M = 32.2, F = 3.48, P = 0.032); tangible support was associated with never been married (M = 16.6, F = 13.6, P < 0.001) and greater financial security (M = 17.8, F = 11.8, P < 0.001); affectionate support showed a significant association with never been married (M = 13.4, F = 12.1, P < 0.001); more financial security (M = 14.1, F = 6.8, P < 0.001); and household income < $35 000 (M = 13.6, F = 5.6, P = 0.004); and positive social interaction was associated with never been married (M = 13.4, F = 2.9, P < 0.001) and annual household income (M = 12.7, F = 5.4, P = 0.005). Lastly, demographic characteristics were also examined in relationship to breast cancer screening patterns. Participants who struggled financially were significantly more likely to belong to the group who were rarely screened X2(4) = 11.9, P = 0.018. No other demographic characteristics were significantly related to breast cancer screening patterns.
Table 2 shows the results of the ANOVA to assess the relationship between overall social support scores and screening patterns. In general, the ANOVA suggests that there is not a statistically significant difference in screening patterns and overall social support score. However, there is a moderately significant relationship between the affectionate support subscale and screening patterns F2,273 = 3.15, P = 0.045). Additional ANCOVA analysis showed that overall social support scores had a statistically significant association with marital status F2,286 = 10.8, P < 0.001; current financial status F2,286 = 14.7, P < 0.001; income F2,286 = 8.0, P < 0.00; and health insurance status F2,286 = 3.3, P = 0.04 (see Table 3).
TABLE 2.
ANOVA of breast cancer screening patterns and MOS score (N = 289)
| Variable | Frequently Screened | Irregularly Screened | Rarely/Never Screened | Range | Test Statistic (df), P value |
|---|---|---|---|---|---|
| Overall social support score | 78.7 (3.8) | 82.2 (3.7) | 75.7 (4.1) | 27–95 | F 2.273 = 2.43, P = 0.090 |
| Social support subscales | |||||
| Emotional/informational | 31.4 (2.0) | 34.5 (1.8) | 33.3 (1.8) | 8–40 | F 2.273 = 2.43, P = 0.090 |
| Tangible support | 15.6 (1.1) | 16.9 (0.9) | 16.1 (1.0) | 3–20 | F2,273 = 1.71, P = 0.183 |
| Affectionate support | 12.4 (0.7) | 13.4 (0.6) | 12.4 (0.7) | 3–15 | F 2,273 = 3.15, P = 0.045 |
| Positive social interaction | 12.5 (0.7) | 13.2 (0.7) | 12.8 (0.7) | 3–15 | F 2,273 = 0.86, P = 0.424 |
Abbreviation: MOS, Medical Outcomes Study.
TABLE 3.
ANCOVA of demographic characteristics and mean social support scores (N = 289)
| Variable | N(%) | Social Support Mean Score (SE) | Range | Test Statistic (df), P values |
|---|---|---|---|---|
| Age | F 1,287 = 0.03, P = 0.867 | |||
| 46 to 55 years | 136 (47) | 73.3 (1.6) | 27–95 | |
| >55 years | 153 (53) | 72.9 (1.5) | 27–95 | |
| Race | F 1,287 = 1.69, P = 0.194 | |||
| Non-white | 8(3) | 81.4 (6.4) | 54–95 | |
| White | 281 (97) | 72.9 (1.1) | 27–95 | |
| Marital status | F 2,286 = 10.8, P < 0.001 | |||
| Married/partnered | 150 (52) | 77.1 (1.4) | 31–95 | |
| Separated/divorced/widowed | 128 (44) | 67.7 (1.6) | 27–56 | |
| Never married | 11(4) | 80.3 (5.3) | 56–95 | |
| Education | F 2,286 = 0.34, P = 0.710 | |||
| Less than high school | 178 (62) | 73.8 (1.4) | 27–95 | |
| High school diploma/GED | 46 (16) | 72.4 (2.7) | 31–95 | |
| Some college | 65 (22) | 71.8 (2.3) | 27–95 | |
| Current financial status | F 2,286 = 14.73, P < 0.001 | |||
| Just about enough | 111 (38) | 76.7 (1.7) | 27–95 | |
| Sometimes struggle | 155 (54) | 68.6 (1.4) | 27–95 | |
| More than needed | 23(8) | 87.1 (3.6) | 51–95 | |
| Income | F 2,286 = 8.0, P < 0.001 | |||
| ≤$25 000 | 201 (60) | 70.9 (1.3) | 27–95 | |
| $25 001 to $35 000 | 33 (11) | 72.3 (3.1) | 31–95 | |
| $35 001 and up | 55 (19) | 81.7 (2.4) | 44–95 | |
| Health insurance | F 2,286 = 3.33, P = 0.037 | |||
| Government | 111 (38) | 72.3 (1.7) | 27–95 | |
| Private/employer | 94 (33) | 76.8 (1.9) | 28–95 | |
| Uninsured | 84 (29) | 70.0 (2.0) | 33–95 |
4 |. DISCUSSION
The purpose of this study was to examine the relationship between social support and breast cancer screening patterns in a sample of rural Appalachian women. Appalachia is well known for its close social relations30 but has been plagued with a high burden of breast cancer mortality and low screening adherence.8,31 Understanding the influence of social support in rural Appalachia communities may be crucial to designing culturally tailored behavioral interventions to promote screening for this vulnerable population.
We found that while the average level of social support is reportedly high, such support was not significantly related to breast cancer screening. There are several possible explanations for why social support and screening are unrelated in this sample. First, the mean social support score was skewed, with most of the sample reporting high social support. This lack of variability and uneven distribution may be related to a ceiling effect, which would limit the applicability of the findings. Still, the literature supports a positive correlation between marital status and social support and an inverse relationship between income and social support, as found in this sample,32 assuring some measure of convergent validity of the scale in this sample.
Another possible explanation is that in this study population, experiencing social support may not be what is needed to promote breast cancer screening. A study by Mair and Thivierge-Rikard,33 of older rural and urban residents and the relationship between social interaction and subjective well-being, found that certain types of interactions between groups have a greater impact on health than others. They found that phone contact with social networks was significantly different from face-to-face visits with friends, relatives, and neighbors, in terms of health promotion. The face-to-face contact was associated with greater subjective well-being.33 In this study, we were not able to distinguish between the type of contact and the frequency of contact that resulted in reports of social support in this population. Understanding mode and frequency of social support may provide additional insight into understanding how social support could be leveraged to promote healthy behaviors in rural communities.
Although social support has been associated with positive health behavior, social support can also be described as harmful or obstructive to performing desired health behaviors.15 Social burden, defined as the negative aspect of social relationships such as caretaking, negative life events, and social strain,34 may provide some explanation for the lack of influence of social support. For example, rural Appalachian women are more likely to share their residence and be a caregiver for elderly parents.35 These extended family networks, while beneficial in some ways, may also have negative effects on health-related behaviors, and further research needs to be conducted to inform behavioral interventions that assuage this barrier.11 In a large national sample of women participating in the Women’s Health Initiative, after controlling for demographic and health characteristics, frequent caregiving was significantly associated with lower likelihood of annual mammography.34 Hence, women who have social burdens are less likely to engage in screening behaviors and adhere to breast cancer screening recommendations.25,34 Social roles are an integral part of rural Appalachian communities and can be an unintended consequence of having a large social network.
Finally, this sample is reflective of the tangible factors that are known to contribute to cancer disparities. The women in the sample lack resources, with approximately one-third being uninsured and more than half of them reporting that they struggle to make ends meet or live in households where the income is $25 000 or less. Income has been consistently linked to low screening rates.25,36 Consistent with the literature, there was a significant association between women in low socioeconomic status and nonadherence to breast cancer screening recommendations. It should be considered that lack of financial security, in addition to having direct effects on the use of cancer screening, might also mitigate the effects of social support on the regular adoption of preventive health behaviors. That is, individuals who lack socioeconomic resources may derive less value from social support than those who have more resources. While this finding does not mitigate the positive effects of social support on health outcomes, it emphasizes the need for interventions that provide connections to both social and structural support for women in this region.
4.1 |. Study limitations
The cross-sectional design of the study prevents the establishment of causality. The findings from this study cannot be generalized to other women in the nation because only rural Appalachian women who were not screened according to recommended guidelines were included in this sample. Finally, our sample was faith-based, which means we possibly excluded women who might have added important depth to the study but were in no way affiliated with a church or willing to attend a church function to enroll in the study. However, attending religious services shapes beliefs about the origins of health and illness, which influences perceptions about its causes and remedies, thereby affecting health care utilization and health behaviors.37
4.2 |. Clinical implications
The picture that emerges from this study is complex, suggesting further research and pointing clearly to the importance of material resources for this community. The most important interventions for this population may be at the individual level. However well designed, interventions will not be as effective without consideration of tangible barriers to screening such as financial status.
These findings do not suggest that social support does not play an important part in the promotion of breast cancer screening. However, these findings should prompt public health researchers to consider social support in the context of the lives of rural Appalachian residents. This exploration should include the negative impact of social networks and the strain produced by extended family networks. Understanding this relationship may allow public health workers to successfully leverage the social ties in the community and the homes of rural Appalachian women to increase screening. Future interventions should focus on understanding the type of social support (eg, positive or obstructive) received by rural Appalachian women who are of age to undergo breast cancer screening and determine how such social support influences adherence to breast cancer screening recommendations. Alternatively, patient navigators can be used in to provide positive social support via culturally appropriate knowledge and comfort to influence uptake of cancer screening practices.38 Concurrently, researchers should address additional barriers to screening among rural Appalachians (eg, transportation and childcare). Lastly, qualitative research methods should be used to provide a comprehensive understanding of the barriers to cancer screening to inform which modifiable barriers should be the focus of future interventions.39
5 |. CONCLUSION
Despite these limitations, the findings can provide important information regarding the development of future interventions to increase mammography among rural Appalachian women based on social support. These data suggest that helping these women find a way to increase mammography screening rates is important. Successful interventions have relied on the use of social networks to promote health behavior changes and cancer screening.40 These findings support the idea that social support is just one factor that influences screening participation and that all social support does not have a positive influence on breast cancer screening patterns. The findings should encourage public health practitioners to go beyond existing measures of social support when designing interventions, being sure to consider multiple influences and to attempt to understand the complex cultural and contextual influences of rural social networks and their overall interaction with health and health-related behaviors.
ACKNOWLEDGMENTS
Funding for this study was provided by the NIH/National Institute of Minority Health and Health Disparities (grant# R24MD002757; PI: Nancy Schoenberg).
Footnotes
DISCLOSURE
The authors have no disclosures to report.
REFERENCES
- 1.Society AC. American Cancer Society guidelines for the early detection of cancer. Breast Cancer 2016; 2017. https://www.cancer.org/healthy/find-cancer-early/cancer-screening-guidelines/American-cancer-society-guidelines-for-the-early-detection-of-cancer.html. Accessed 5/30/2017. [Google Scholar]
- 2.McCarthy EP, Burns RB, Freund KM, et al. Mammography use, breast cancer stage at diagnosis, and survival among older women. J Am Geriatr Soc. 2000;48(10):1226–1233. [DOI] [PubMed] [Google Scholar]
- 3.Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2009;151(10):716. [DOI] [PubMed] [Google Scholar]
- 4.Yao N, Alcala HE, Anderson R, Balkrishnan R. Cancer disparities in rural Appalachia: incidence, early detection, and survivorship. J Rural Health. 2016. 10.1111/jrh.12213 [DOI] [PubMed] [Google Scholar]
- 5.Age-adjusted cancer mortality rates by Appalachian region in Kentucky-BodrJKCRC-RiRJ, 2017, from http://cancer-rates.info/ky/.
- 6.Gøtzsche PC, Jørgensen KJ. Screening for breast cancer with mammography. Cochrane Libr. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21(1):13–20. [DOI] [PubMed] [Google Scholar]
- 8.Knight JR, Huang B, Tucker T. Breast cancer in Kentucky: progress and possibilities. Dr Alvarado Goes to Frankfort 2015:93. [Google Scholar]
- 9.Anderson RT, Yang T-C, Matthews SA, et al. Breast cancer screening, area deprivation, and later-stage breast cancer in Appalachia: Does geography matter? Health Serv Res. 2014;49(2):546–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGarvey EL, Leon-Verdin M, Killos LF, Guterbock T, Cohn WF. Health disparities between Appalachian and non-Appalachian counties in Virginia USA. J Community Health. 2011;36(3):348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz ML, Reiter PL, Young GS, Pennell ML, Tatum CM, Paskett ED. Adherence to multiple cancer screening tests among women living in Appalachia Ohio. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health UDo, Services H. Healthy People 2020 Topics and Objectives: Cancer. Washington, DC: US Department of Health and Human Services:2011. [Google Scholar]
- 13.Thompson T, Rodebaugh TL, Pérez M, et al. Influence of neighborhood-level factors on social support in early-stage breast Cancer patients and controls. Soc Sci Med. 2016;156:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson T, Rodebaugh TL, Pérez M, Schootman M, Jeffe DB. Perceived social support change in patients with early-stage breast cancer and controls. Health Psychol. 2013;32(8):886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardach SH, Tarasenko YN, Schoenberg NE. The role of social support in multiple morbidity: self-management among rural residents. J Health Care Poor Underserved. 2011;22(3):756–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaney C, Israel BA. Chapter 9: Social Networks and Social Support. In: Glanz KRB, Viswanath K, eds. Health Behavior and Health Education: Theory, Research, and Practice. 4th ed. San Francisco, CA: Jossey-Bass; 2008:189–210. [Google Scholar]
- 17.Magai C, Consedine N, Neugut AI, Hershman DL. Common psychosocial factors underlying breast cancer screning and breast cancer treatment adherence: a conceptual review and synthesis. J Womens Health. 2007;16(1):11–23. [DOI] [PubMed] [Google Scholar]
- 18.Berkman LF, Glass T, Birssette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Soc Sci Med. 2000;51(6):843–857. [DOI] [PubMed] [Google Scholar]
- 19.Maxwell CJ, Kozak JF, Desjardins-Denault SD, Parboosingh J. Factors important in promoting mammography screening among Canadian women. Can J Public Health. 1997;88(5):346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexandraki I, Mooradian AD. Barriers related to mammography use for breast cancer screening among minority women. J Natl Med Assoc. 2010;102(3):206–218. [DOI] [PubMed] [Google Scholar]
- 21.Baldaro B, Surcinelli P, Rossi A, Fasol R, Mazzetti M, Bolzani R. Short communication: Investigation into factors that promote adherence to a mammography screening programme. Stress Health. 2007;23(5):277–283. [Google Scholar]
- 22.Jones RA, Steeves R, Williams I. Family and friend interactions among African-American men deciding whether or not to have a prostate cancer screening. Urol Nurs. 2010;30(3):189–194. [PMC free article] [PubMed] [Google Scholar]
- 23.Agriculture USDo. 2017. https://www.ers.usda.gov/data-products/atlas-of-rural-and-small-town-america/go-to-the-atlas/. Accessed July 16, 2017.
- 24.Schoenberg N, Hatcher J, Dignan MB, Shelton B, Wright S, Dollarhide KF. Faith moves mountains: an Appalachian cervical cancer prevention program. Am J Health Behav. 2009;33(6):627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatcher J, Studts CR, Dignan MB, Turner LM, Schoenberg NE. Predictors of cervical cancer screening for rarely or never screened rural Appalachian women. J Health Care Poor Underserved. 2011;22:175–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. [DOI] [PubMed] [Google Scholar]
- 27.Vyavaharkar M, Moneyham L, Tavakoli A, et al. Social support, coping, and medication adherence among HIV-positive women with depression living in rural areas of the southeastern United States. AIDS Patient Care STDS. 2007;21(9):667–680. [DOI] [PubMed] [Google Scholar]
- 28.Logan TK, Walker R, Cole J, Ratliff S, Leukefeld C. Qualitative differences among rural and urban intimate violence victimization experiences and consequences: a pilot study. J Fam Violence. 2003;18(2):83–92. [Google Scholar]
- 29.Appalachian Regional Commision. Demographic and health information. 2008. [Google Scholar]
- 30.Helton LR, Keller SM. Appalachian women: a study of resiliency assets and cultural values. J Soc Serv Res. 2010;36(2):151–161. [Google Scholar]
- 31.Nguyen-Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol. 2014;24(3):228–235. [DOI] [PubMed] [Google Scholar]
- 32.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23(2):207–218. [DOI] [PubMed] [Google Scholar]
- 33.Mair CA, Thivierge-Rikard RV. The strength of strong ties of older rural adults: regional distinctions in the relationship between social interaction and subjective well-being. Int J Aging Hum Dev. 2010;70(2):119–143. [DOI] [PubMed] [Google Scholar]
- 34.Messina CR, Lane DS, West DS, et al. Relationship of social support and social burden to repeated breast cancer screening in the Women’s Health Initiative. Health Psychol. 2004;23(6):582–594. [DOI] [PubMed] [Google Scholar]
- 35.Mather M. Households and Families in Appalachia. Washington: Appalachian Regional Commission; 2004. [Google Scholar]
- 36.Hatcher J, Rayens MK, Schoenberg NE. Mammography promotion in the emergency department: a pilot study. Public Health Nurs. 2010;27(6):520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen JD, Leyva B, Torres A, et al. Religious beliefs and cancer screening behaviors among Catholic Latinos: implications for faith-based interventions. J Health Care Poor Underserved. 2014;25(2):503–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paskett ED, Llanos AA, Young GS, Pennell ML, Lee CJ, Katz ML. Correlates of colorectal cancer screening among residents of Ohio Appalachia. J Community Health. 2013;38(4):609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paskett ED, Fisher JL, Lengerich EJ, et al. Disparities in underserved white populations: the case of cancer-related disparities in Appalachia. Oncologist. 2011;16(8):1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latkin CA, Knowlton AR. Social network assessments and interventions for health behavior change: a critical review. Behav Med. 2015;41(3):90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


