Key Points
We demonstrate risk factors for HCoV LRTI in allogeneic HCT recipients and significance of virologic documentation by BAL on mortality.
Hyperglycemia associated with steroid use appears to be a strong predictor of HCoV disease progression.
Abstract
Data are limited regarding risk factors for lower respiratory tract infection (LRTI) caused by seasonal human coronaviruses (HCoVs) and the significance of virologic documentation by bronchoalveolar lavage (BAL) on outcomes in hematopoietic cell transplant (HCT) recipients. We retrospectively analyzed patients undergoing allogeneic HCT (4/2008-9/2018) with HCoV (OC43/NL63/HKU1/229E) detected by polymerase chain reaction during conditioning or post-HCT. Risk factors for all manifestations of LRTI and progression to LRTI among those presenting with HCoV upper respiratory tract infection (URTI) were analyzed by logistic regression and Cox proportional hazard models, respectively. Mortality rates following HCoV LRTI were compared according to virologic documentation by BAL. A total of 297 patients (61 children and 236 adults) developed HCoV infection as follows: 254 had URTI alone, 18 presented with LRTI, and 25 progressed from URTI to LRTI (median, 16 days; range, 2-62 days). Multivariable logistic regression analyses showed that male sex, higher immunodeficiency scoring index, albumin <3 g/dL, glucose >150 mg/dL, and presence of respiratory copathogens were associated with occurrence of LRTI. Hyperglycemia with steroid use was associated with progression to LRTI (P < .01) in Cox models. LRTI with HCoV detected in BAL was associated with higher mortality than LRTI without documented detection in BAL (P < .01). In conclusion, we identified factors associated with HCoV LRTI, some of which are less commonly appreciated to be risk factors for LRTI with other respiratory viruses in HCT recipients. The association of hyperglycemia with LRTI might provide an intervention opportunity to reduce the risk of LRTI.
Visual Abstract
Introduction
Novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is currently circulating worldwide, causing significant morbidity and mortality.1 Seasonal human coronaviruses (HCoVs) are already known to be ubiquitous and recognized as respiratory pathogens in humans, typically causing mild respiratory illness in immunocompetent individuals.2 Limited data suggest that detection of HCoVs in lower respiratory tract specimens is associated with high rates of mortality in hematopoietic cell transplantation (HCT) recipients.3 However, risk factors for progression to lower respiratory tract infection (LRTI) among patients who presented with HCoV upper respiratory tract infection (URTI) and the significance of HCoV detection in bronchoalveolar lavage (BAL), indicating lower respiratory tract involvement, are rarely systematically evaluated in this high-risk population.4,5 Understanding these features of HCoVs is crucial when evaluating the significance of SARS-CoV-2 in transplant recipients.6 The objective of this study was to identify risk factors for HCoV LRTI in allogeneic HCT recipients and investigate whether outcomes differ among patients with LRTI according to virologic documentation of lower respiratory tract involvement by BAL.
Methods
Study design
We reviewed allogeneic HCT recipients whose first HCoV infection was documented during conditioning or after transplantation through June 2019 to identify risk factors for LRTI.7 The transplant recipients were identified from 2 cohorts at the Fred Hutchinson Cancer Research Center (Fred Hutch). The first cohort included patients who underwent transplantation between July 2009 and September 2018 and had respiratory tract samples collected and tested for clinical purposes. The second cohort was a subset of patients from a prospective surveillance study of allogeneic HCT recipients undergoing transplantation from April 2008 to February 2010 in which standardized respiratory symptom surveys and multiplex respiratory polymerase chain reaction (PCR) tests were performed regardless of symptoms at several timepoints: pre-HCT, weekly during the first 100 days post-HCT, and at least every 3 months through 1 year post-HCT.8 Clinical samples were also collected at clinicians’ discretion if respiratory symptoms were noted. For the current study, we included subjects with respiratory symptoms at the time of first detection of HCoV. For evaluation of outcome following LRTI according to virologic documentation by BAL, we included all transplant recipients with first HCoV LRTI. Demographic and clinical data were extracted from Fred Hutch’s database and medical chart review. The study was approved by the Institutional Review Board at Fred Hutch and was conducted in accordance with the Declaration of Helsinki.
Laboratory testing
Upper (nasopharyngeal and nasal wash) and lower (BAL) respiratory tract samples were tested by multiplex semiquantitative, reverse-transcription PCR for 12 respiratory viruses. This laboratory developed assay detects all 4 species of seasonal HCoVs (OC43/NL63/HKU1/229E); however, strain-specific PCR is not routinely performed.8-10 All PCR reactions were performed according to College of American Pathologists standards. Some pediatric transplant recipients underwent a commercial multiplex qualitative respiratory PCR assay (FilmArray; BioFire Diagnostics, Salt Lake City, UT) for an initial diagnosis, after which HCoV was evaluated by the laboratory developed test for quantification.11 Institutional standard investigation of BAL specimens includes broad diagnostic tests, including multiplex PCR for respiratory viruses; conventional cultures for bacteria, fungi, mycobacteria, and viruses; shell vial culture for cytomegalovirus; immunofluorescent antibody staining for Pneumocystis jirovecii; Aspergillus galactomannan enzyme-linked immunosorbent assay; cytopathologic examination; and PCR for Legionella and fungus.3
Definitions
URTI was defined as HCoV detection in an upper respiratory tract sample with respiratory symptoms. As previously described, proven or probable LRTI was defined as having virus detected from a lower respiratory tract sample (BAL) with or without new pulmonary infiltrates by chest radiography, respectively.12 Possible LRTI was defined as having virus detected from an upper respiratory tract sample with new pulmonary infiltrates (but without confirmation of virus in a lower respiratory tract sample). Patients who met criteria for LRTI within 1 day of URTI were considered to have LRTI at presentation and were not included in the LRTI progression analysis.7 A HCoV illness event was considered to be a new event if ≥12 weeks elapsed between 2 positive samples or if there were ≥2 negative HCoV samples between 2 HCoV-positive samples.13 A respiratory copathogen was defined as a pathogen detected in a concurrent respiratory sample. A copathogen in blood was defined as a pathogen or antigen (bacteria, fungi, virus, or Aspergillus galactomannan enzyme-linked immunosorbent assay) detected in a blood sample obtained within 2 days of diagnosis of HCoV infection.12 Nearest values of blood cell counts and serum glucose within 2 weeks before HCoV diagnosis were recorded. Similarly, lowest serum albumin value and highest daily steroid dose within 2 weeks prior to HCoV diagnosis were collected. Graft-versus-host disease (GVHD) grades represent maximum grades. GVHD severity at the time of HCoV infection was assessed by the highest daily corticosteroid dose administered within 2 weeks prior to the diagnosis of HCoV infection. Glucose values were categorized as most recent glucose value >150 mg/dL, ≤150 mg/dL, or missing.14 The variable of most recent glucose value >150 mg/dL was further subcategorized according to whether the high glucose values were observed repeatedly or not within 2 weeks before HCoV diagnosis.
Statistical analysis
Patient characteristics were compared among disease categories using χ2 or Fisher’s exact test for categorical variables and Student t test or Wilcoxon rank-sum test for continuous variables (as appropriate). The probability of progression to HCoV LRTI among patients who presented with HCoV URTI was estimated by cumulative incidence curves, treating death as a competing risk. Gray’s test was used to compare cumulative incidence probabilities between categories. Cox proportional hazards models were used to estimate unadjusted and adjusted hazard ratios (HRs) for progression to HCoV LRTI within 90 days of HCoV URTI. Logistic regression models were used to evaluate cross-sectional association between each risk factor and occurrence of LRTI among all patients with first HCoV infection (including patients who presented with HCoV LRTI). Overall survival rates following HCoV LRTI were compared according to virologic documentation by BAL (log-rank test).
All covariates with P values < 0.1 in the univariable analyses were candidates for inclusion in the multivariable models. Immunodeficiency scoring index (ISI) was originally developed to predict progression to respiratory syncytial virus (RSV) LRTI as a discreate variable in transplant recipients.15 ISI was treated as a continuous variable in multivariable models given a limited number of outcome events, and steroid use and blood cell counts were not included in the same models with ISI, as these are components of ISI.15 For multivariable Cox regression models, serum glucose levels were correlated with steroid use, and it was not feasible to calculate each effect given the sample size. Therefore, we created a composite variable for glucose and steroid use to evaluate the joint effects. For multivariable logistic regression models, we also performed a sensitivity analysis where to evaluate whether including steroid use and cell counts instead of ISI provided a better-fitting model. Two-sided P values < .05 were considered statistically significant. All statistical analyses were performed using SAS 9.4 for Windows (SAS Institute, Cary, NC).
Results
Patient characteristics
Among 2553 patients undergoing allogeneic HCT during the study period, 297 patients (61 children and 236 adults) had documented symptomatic HCoV infection (supplemental Figure 1). Characteristics of each HCoV infection group are shown in Table 1. The majority of first HCoV infections occurred after day 30 following HCT (254/297, 86%) with a median of 174 days and a range of −9 to 3489 days relative to the date of HCT. Among patients with first HCoV infection, 254 had URTI alone, 18 presented with LRTI, and 25 progressed from URTI to LRTI (progression rate of 9% after a median of 16 days; range, 2-62 days). Among 254 patients who had URTI only during first HCoV infection episodes, 10 patients developed LRTI during the subsequent HCoV infection episodes. The days from the first episode and subsequent episode of those 10 patients were as follows: median of 549 days, range of 38 days to 795 days. A total of 53 patients (2.1%) were found to have HCoV LRTI (16 proven/probable LRTI and 37 possible LRTI) in this entire cohort.
Table 1.
Characteristics of allogeneic HCT recipients with first documented HCoV infection (N = 297)
| Characteristics | Categories | Total (N = 297) | URTI (N = 254) | LRTI (N = 43) |
|---|---|---|---|---|
| Age at HCoV diagnosis, y | Median (IQR) | 45 (25-60) | 44 (23-60) | 48 (34-62) |
| Children at HCoV diagnosis | <18 y old | 61 (21) | 55 (22) | 6 (14) |
| Children’s age at HCoV diagnosis, y | Median (range) | 9 (0-18) | 9 (0-18) | 5 (0-18) |
| Sex | Male | 170 (57) | 140 (55) | 30 (70) |
| Female | 127 (43) | 114 (45) | 13 (30) | |
| Race | White | 239 (80) | 206 (81) | 33 (77) |
| Nonwhite | 42 (14) | 37 (15) | 5 (12) | |
| Others | 16 (5) | 11 (4) | 5 (12) | |
| Year of transplant | 2008-2013 | 141 (47) | 123 (48) | 18 (42) |
| 2014-2018 | 156 (53) | 131 (52) | 25 (58) | |
| Transplant number | First | 238 (80) | 206 (81) | 32 (74) |
| Second or higher | 59 (20) | 48 (19) | 11 (26) | |
| Stem cell source | Bone marrow | 52 (18) | 46 (18) | 6 (14) |
| PBSC | 206 (69) | 177 (70) | 29 (67) | |
| Cord blood | 39 (13) | 31 (12) | 8 (19) | |
| Human leukocyte antigen-match/donor type | Matched/unrelated | 128 (43) | 113 (44) | 15 (35) |
| Mismatch/related or unrelated | 32 (11) | 28 (11) | 4 (9) | |
| Haplo-/related | 23 (8) | 16 (6) | 7 (16) | |
| Matched/related | 75 (25) | 66 (26) | 9 (21) | |
| Cord/unrelated | 39 (13) | 31 (12) | 8 (19) | |
| Recipient blood type | O+ or O− | 137 (46) | 113 (44) | 24 (56) |
| A+ or A− | 109 (36) | 94 (37) | 15 (35) | |
| B+ or B− | 37 (12) | 34 (13) | 3 (7) | |
| AB+ or AB− | 10 (3) | 10 (4) | 0 (0) | |
| Missing | 1 (1) | 3 (1) | 1 (2) | |
| Donor blood type | O+ or O− | 123 (41) | 105 (42) | 18 (42) |
| A+ or A− | 105 (35) | 93 (37) | 12 (28) | |
| B+ or B− | 32 (11) | 28 (11) | 4 (9) | |
| AB+ or A− | 10 (4) | 8 (3) | 2 (5) | |
| Missing | 27 (9) | 20 (8) | 7 (16) | |
| Conditioning regimen | Myeloablative | 192 (65) | 163 (64) | 29 (67) |
| Nonmyeloablative | 105 (35) | 91 (36) | 14 (33) | |
| Smoking status | Current | 1 (0) | 1 (0) | 0 (0) |
| Former | 67 (23) | 57 (22) | 10 (23) | |
| Never | 228 (77) | 195 (77) | 33 (77) | |
| Missing | 1 (0) | 1 (0) | 0 (0) | |
| Body habitus* | Normal or underweight | 135 (45) | 119 (47) | 16 (37) |
| Overweight | 88 (30) | 72 (28) | 16 (37) | |
| Obese | 74 (25) | 63 (25) | 11 (26) | |
| Diabetic state at HCT | Yes | 20 (7) | 17 (7) | 3 (7) |
| No | 216 (73) | 184 (72) | 32 (74) | |
| Missing | 61 (21) | 53 (21) | 8 (19) | |
| Recipient CMV status | Seropositive | 197 (66) | 167 (66) | 30 (70) |
| Seronegative | 100 (34) | 87 (34) | 13 (30) | |
| Donor CMV status | Seropositive | 126 (42) | 111 (44) | 15 (35) |
| Seronegative | 171 (58) | 143 (56) | 28 (65) | |
| Days between date of HCT and onset of HCoV infection | ≤30 | 43 (14) | 37 (15) | 6 (14) |
| 31-365 | 158 (53) | 133 (52) | 25 (58) | |
| >365 | 96 (32) | 84 (33) | 12 (28) | |
| Days between date of HCT and onset of HCoV infection | ≤Lower quartile (57) | 71 (24) | 63 (25) | 8 (19) |
| Lower quartile (57) to median (155) | 73 (25) | 63 (25) | 10 (23) | |
| Median (155) to upper quartile (474) | 78 (26) | 61 (24) | 17 (40) | |
| >Upper quartile (474) | 75 (25) | 67 (26) | 8 (19) | |
| Acute GVHD | Grade 0-1 | 83 (28) | 69 (27) | 14 (33) |
| Grade 2 | 179 (60) | 158 (62) | 21 (49) | |
| Grade 3-4 | 35 (12) | 27 (11) | 8 (19) | |
| Chronic GVHD | Yes | 286 (96) | 246 (97) | 40 (93) |
| No | 11 (4) | 8 (3) | 3 (7) | |
| HCoV species | OC43 | 6 (2) | 2 (1) | 4 (9) |
| NL63 | 4 (1) | 4 (2) | 0 (0) | |
| HKU1 | 4 (1) | 2 (1) | 2 (5) | |
| 229E | 3 (1) | 2 (1) | 1 (2) | |
| Missing | 280 (94) | 244 (96) | 36 (84) | |
| HCoV Ct values† | Median (IQR) | 26 (23-31) | 26 (23-31) | 28 (26-30) |
| Copathogen in upper respiratory tractठ| Yes | 67 (23) | 52 (20) | 15 (35) |
| No | 230 (7) | 202 (80) | 28 (65) | |
| Copathogen in blood||¶ | Yes | 37 (12) | 28 (11) | 9 (21) |
| No | 260 (88) | 226 (89) | 34 (79) | |
| ISI‡ | Low (0-2) | 66 (22) | 65 (26) | 1 (2) |
| Moderate (3-6) | 208 (70) | 172 (68) | 36 (84) | |
| High (7-11) | 23 (8) | 17 (7) | 6 (14) | |
| Neutrophil count# | ≤500 × 106 cells/L | 24 (8) | 18 (7) | 6 (14) |
| >500 × 106 cells/L | 248 (84) | 217 (85) | 31 (72) | |
| Missing | 25 (8) | 19 (7) | 6 (14) | |
| Lymphocyte count# | ≤300 × 106 cells/L | 44 (15) | 37 (15) | 7 (16) |
| >300 × 106 cells/L | 226 (76) | 196 (77) | 30 (70) | |
| Missing | 27 (9) | 21 (8) | 6 (14) | |
| ≤200 × 106 cells/L | 30 (10) | 25 (10) | 5 (12) | |
| >200 × 106 cells/L | 240 (81) | 208 (82) | 32 (74) | |
| ≤100 × 106 cells/L | 15 (5) | 12 (5) | 3 (7) | |
| >100 × 106 cells/L | 255 (86) | 221 (87) | 34 (79) | |
| Monocyte count# | ≤300 × 106 cells/L | 77 (26) | 61 (24) | 16 (37) |
| >300 × 106 cells/L | 193 (65) | 172 (68) | 21 (49) | |
| Missing | 27 (9) | 21 (8) | 6 (14) | |
| ≤200 × 106 cells/L | 48 (16) | 36 (14) | 12 (28) | |
| >200 × 106 cells/L | 222 (75) | 197 (78) | 25 (58) | |
| ≤100 × 106 cells/L | 32 (11) | 22 (9) | 10 (23) | |
| >100 × 106 cells/L | 238 (80) | 211 (83) | 27 (63) | |
| Albumin** | ≤3 g/dL | 51 (17) | 35 (14) | 16 (37) |
| >3 g/dL | 214 (72) | 189 (74) | 25 (58) | |
| Missing | 32 (11) | 30 (12) | 2 (5) | |
| Glucose# | 0-100 mg/dL | 73 (25) | 60 (24) | 13 (31) |
| 101-150 mg/dL | 127 (43) | 118 (46) | 9 (21) | |
| 151-200 mg/dL | 35 (12) | 24 (9) | 11 (26) | |
| >200 mg/dL | 14 (4) | 10 (4) | 4 (10) | |
| Missing | 48 (16) | 42 (17) | 6 (14) | |
| Glucose†† | ≤150 mg/dL or unknown | 195 (66) | 172 (68) | 23 (53) |
| >150 mg/dL on most recent day only | 18 (6) | 13 (5) | 5 (12) | |
| >150 mg/dL on most recent day and >150 mg/dL on another day | 31 (10) | 21 (8) | 10 (23) | |
| Any glucose value other than most recent >150 mg/dL | 53 (18) | 48 (19) | 5 (12) | |
| Steroid dose‡‡ | None | 134 (45) | 122 (48) | 12 (28) |
| <1 mg/kg | 136 (46) | 111 (44) | 25 (58) | |
| ≥1 mg/kg | 27 (9) | 21 (8) | 6 (14) | |
| HbA1c§§ | <6.5% | 34 (11) | 27 (11) | 7 (16) |
| ≥6.5% | 15 (5) | 12 (5) | 3 (7) | |
| Missing | 248 (84) | 215 (85) | 33 (77) |
Values are n (%) unless otherwise specified.
CMV, cytomegalovirus; Ct, cycle threshold; IQR, interquartile range, PBSC, peripheral blood stem cell.
Overweight is defined as a body mass index ≥25 and <30 for adults and ≥85th percentile and <95th percentile for children of the same age and sex. Obesity is defined as a body mass index ≥30 for adults and ≥95th percentile for children of the same age and sex according to the Centers for Disease Control and Prevention.
Missing values exist.
At HCoV diagnosis.
Copathogens: human rhinovirus 21, RSV 17, parainfluenza virus 14, human metapneumovirus 9, human bocavirus 8, influenza virus 6, adenovirus 5 (the total number of copathogens are larger than that of patients with copathogens detected, since some patients had >1 pathogen).
Defined as a pathogen or antigen (bacteria, fungi, virus, Aspergillus galactomannan enzyme-linked immunosorbent assay) detected in a blood within 2 d of HCoV diagnosis.
Copathogens: cytomegalovirus (any positive) 27, adenovirus 3, Aspergillus galactomannan enzyme-linked immunosorbent assay 2, Candida albicans 1, Clostridium septicum 1, Viridans Streptococcus 1, Hafnia alvei 1, EBV 1, human herpes virus 6 1, and BK virus 1 (the total number of copathogens are larger than that of patients with copathogens detected since some patients had >1 pathogens).
Using nearest value within 2 weeks before HCoV diagnosis.
Lowest albumin value in the 2 weeks before HCoV diagnosis.
Using values within 2 weeks before HCoV diagnosis.
Highest daily steroid dose in the 2 weeks before HCoV diagnosis.
Highest HbA1c within 3 months before HCoV diagnosis.
Risk factors for occurrence of LRTI
In univariable logistic regression models, all variables shown in supplemental Table 1 were evaluated to identify factors associated with occurrence of LRTI. In multivariable models including ISI as a continuous variable, male sex, higher ISI, albumin level <3 g/dL, glucose value >150 mg/dL, and presence of respiratory copathogens at HCoV diagnosis were associated with LRTI (Figure 1A). Although steroid use and monocytopenia were associated with LRTI in univariable models, these were not included in above adjusted models, since cytopenia and steroid use are components of ISI (as described in “Methods”). Therefore, we also performed additional multivariable analyses to evaluate the independent effects of steroid use and monocytopenia (instead of ISI); only hypoalbuminemia (<3 g/dL) and hyperglycemia (>150 mg/dL) remained significant (Figure 1B).
Figure 1.
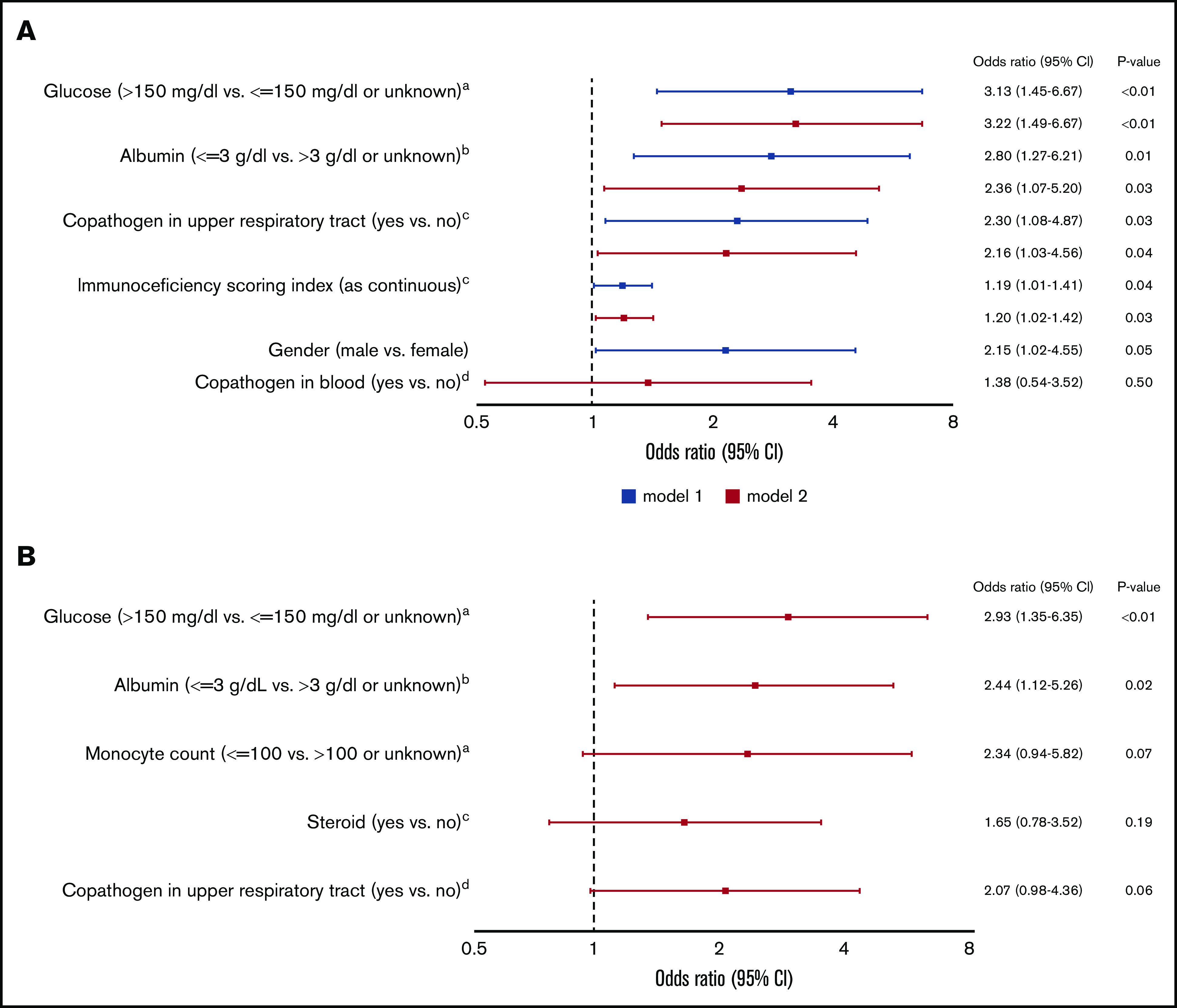
Multivariable logistic regression models for occurrence of HCoV LRTI among 297 patients with first HCoV infection (43 outcome events). (A) Models including ISI. aNearest value within 2 weeks before HCoV diagnosis. bLowest albumin level in the 2 weeks before HCoV diagnosis. cAt HCoV diagnosis. dA pathogen or antigen detected in a blood within 2 days of HCoV diagnosis. (B) Models including steroid use and monocyte count instead of ISI. aNearest value within 2 weeks before HCoV diagnosis. bLowest albumin level in the 2 weeks before HCoV diagnosis. cHighest daily steroid dose in the 2 weeks before HCoV diagnosis. dAt HCoV diagnosis. CI, confidence interval; OR, odds ratio.
Risk factors for progression to LRTI
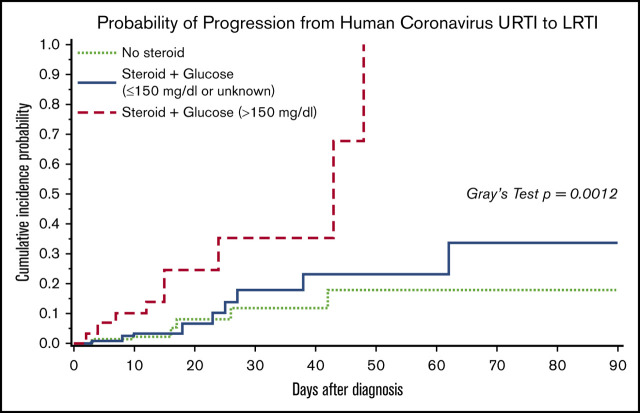
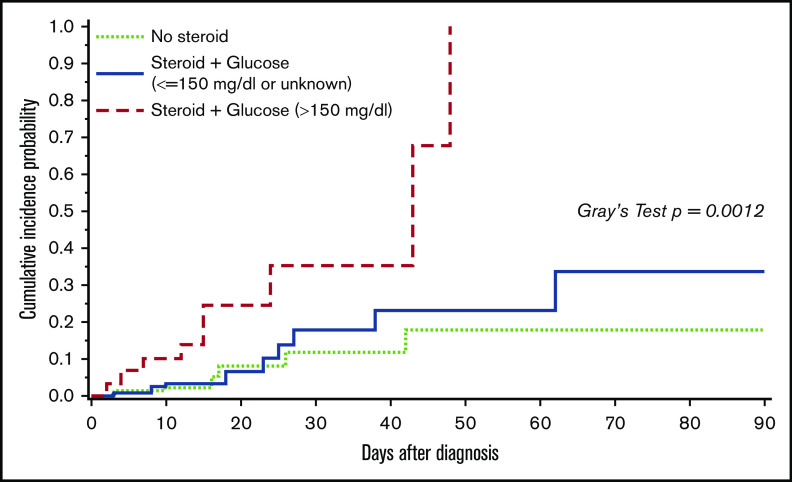
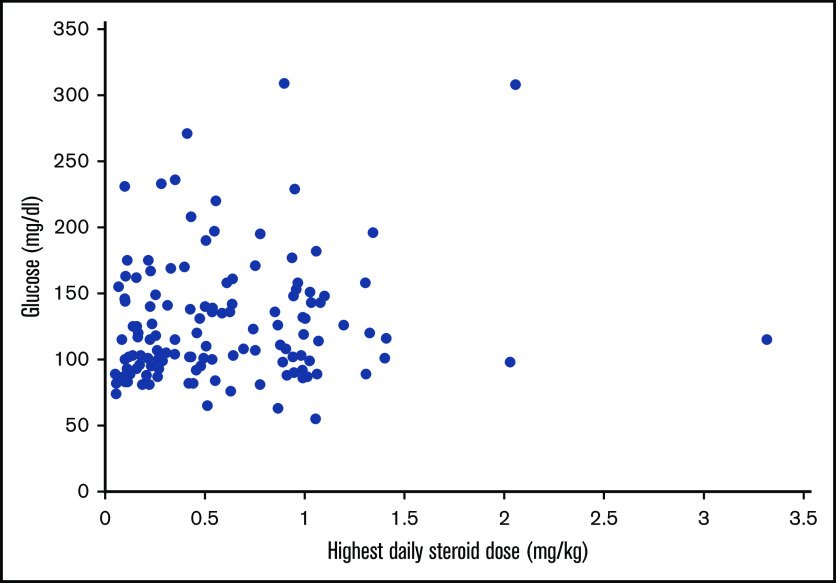
Univariable analyses for progression to HCoV LRTI revealed risk-factor candidates, including transplant number, ISI, steroid dose, and glucose value >150 mg/dL (supplemental Table 1). Given the high collinearity between steroid use and hyperglycemia and the limited number of events, a composite variable for glucose and steroid was included into multivariable models to evaluate the effect of both factors. Models consistently demonstrated significant association of hyperglycemia with steroid use with progression to LRTI (Table 2). Figure 2 shows the cumulative incidence curve of progression to LRTI stratified by the categories of the composite variable. The probability of progression to LRTI in patients with hyperglycemia and steroid use at the time of HCoV URTI reached 100% at day 48 (P < .01 with Gray’s test). Furthermore, we examined the relationship between highest daily steroid dose (mg/kg) and glucose value (mg/dL) among 130 patients who had systemic steroid use in the 2 weeks prior to HCoV URTI (Figure 3), and the correlation was weak (R2 = 0.13). Of note, higher viral load (lower Ct value) was not associated with increased risk of occurrence of or progression to LRTI (supplemental Table 1).
Table 2.
Multivariable cox regression models for progression to HCoV LRTI
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Covariates | Categories | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Combined steroid and glucose, mg/dL*† | Steroid + glucose (>150) vs no steroid | 4.73 (1.60-14.0) | <.01 | 4.83 (1.61-14.5) | <.01 | ||
| Steroid + glucose (≤150 or unknown) vs no steroid | 1.51 (0.58-3.98) | .4 | 1.44 (0.54-3.84) | .47 | |||
| Transplant number | Second or higher HCT vs first HCT | 1.56 (0.63-3.90) | .34 | 2.17 (0.92-5.09) | .08 | ||
| ISI‡ | As continuous | 1.07 (0.89-1.28) | .47 | 1.14 (0.97-1.34) | .12 | ||
Total of 25 LRTI events among 279 patients presenting with first HCoV URTI.
Systemic steroids in the 2 weeks before HCoV diagnosis.
Using nearest value within 2 weeks before HCoV diagnosis.
At HCoV diagnosis.
Figure 2.
Cumulative incidence of progression to HCoV LRTI by day 90 among 279 patients presenting with first HCoV URTI. P value is < .01 with Gray’s tests. Among 129 patients with no steroid, none of 13 patients with glucose values >150 mg/dL progressed to LRTI and 7 of 116 (6%) patients with glucose values ≤150 mg/dL or unknown progressed to LRTI. With the limited sample size, we combined those as a group of no steroid use.
Figure 3.
Relationship between glucose value and steroid dose. Scatterplots depict the relationship between highest daily steroid dose (mg/kg) and glucose value (mg/dL) among 130 patients who received systemic steroids in 2 weeks prior to human coronavirus URTI (R2 = 0.13). Glucose was the nearest within 2 weeks before URTI.
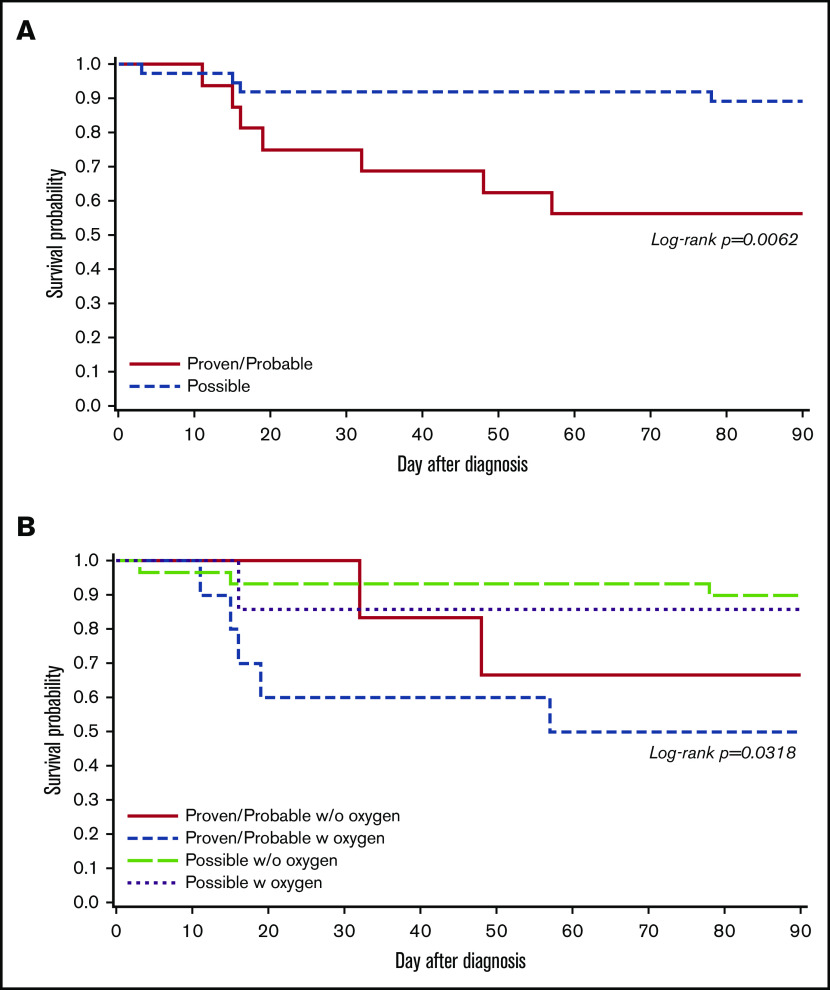
Outcomes following proven/probable vs possible LRTI
The probabilities of overall survival at 90 days following proven/probable and possible LRTI are shown in Figure 4A (log-rank test, P < .01). The outcomes following proven/probable LRTI were worse than possible LRTI, and the trend appears to be consistent after stratifying groups according to oxygen use at the time of LRTI diagnosis (Figure 4B; log-rank test, P = .03). Among 16 patients with proven/probable LRTI, 8 patients were found to have respiratory copathogens such as RSV, human metapneumovirus, rhinovirus, Staphylococcus aureus, Elizabethkingia, and Aspergillus fumigatus, and 3 of 8 patients with copathogens died within 90 days of LRTI diagnosis. Similarly, 4 of 8 patients who had no respiratory copathogen died within 90 days.
Figure 4.
Overall survival by day 90 according to HCoV LRTI categories among patients with first HCoV LRTI (N = 53). (A) Kaplan-Meier estimate of overall survival by LRTI categories (proven/probable vs possible LRTI; P < .01). (B) Kaplan-Meier estimate of overall survival according to LRTI categories (proven/probable vs possible LRTI) with oxygen requirement at LRTI diagnosis (P = .03).
Discussion
This study demonstrated that hypoalbuminemia, male sex, high serum glucose, presence of respiratory copathogens, and higher ISI were associated with the occurrence of HCoV LRTI. Hyperglycemia frequently occurred in the context of steroid use and appeared to be unrelated to steroid dose. Steroids with hyperglycemia did increase the risk of progression to LRTI among patients who presented with HCoV URTI, but steroids without hyperglycemia did not. Mortality rates following proven/probable HCoV LRTI were higher than possible LRTI.
A novel finding is that hyperglycemia is an important risk factor for occurrence of LRTI based on the multivariable logistic regression models. Similarly, Cox regression models using a composite variable of glucose value and steroid use consistently demonstrated a significant association between hyperglycemia with steroid use and progression from URTI to LRTI (Table 2). Prior studies in HCT recipients suggested that steroid use and active GVHD status were associated with occurrence of HCoV LRTI; however, steroid dose and serum glucose level were not evaluated.4,5 Systemic corticosteroid use, standard therapy for active GVHD, can induce hyperglycemia, and higher doses of steroid use are a known risk factor for progression to LRTI with other respiratory viruses in transplant recipients; therefore, we hypothesized that the effect of hyperglycemia primarily reflects the impact of steroid dose.7,13,16 However, there was only a weak correlation between steroid dose and glucose value (Figure 3). Thus, it appears that glucose values were independent of steroid dose and hyperglycemia in the context of steroid use is a risk factor for progression to LRTI.
Whether hyperglycemia as a risk factor for respiratory viral disease progression in immunocompromised hosts is poorly understood. Jung et al reported that among cancer patients who received steroids as part of induction therapy, steroid-induced hyperglycemia was associated with serious infection (bacterial and fungal infection).17 A recent study indicated that hyperglycemia (each 10-mg/dL increase in glucose level) was associated with increased risk of viremia/viruria in transplant recipients.18 Hyperglycemia has been recognized as a prognostic factor for poor outcomes in patients with SARS-CoV-2 regardless of the previous history of diabetes, and routine screening of hyperglycemia has been proposed for hospitalized patients with SARS-CoV-2.19-21 Several clinical studies suggested that patients with diabetes are at risk for severe H1N1 influenza infections.22 In an animal model, diabetic mice had increased susceptibility to severe infections with influenza virus, and the enhanced susceptibility was reversed with insulin.23 Hyperglycemia affects innate immunity in various ways, and the exact mechanisms remain to be elucidated.24,25 Recent data highlighted the role of increased glucose metabolism in influenza A virus–induced cytokine storm; influenza A virus promotes O-linked β-N-acetylglucosamine transferase binding to interferon regulatory factor 5, leading to subsequent downstream inflammatory cytokine production.26 The role of hyperglycemia with or without steroid use as well as glycemic control on progression to LRTI due to HCoV or other respiratory viruses should be further evaluated.
The role of HCoV infection in immunocompromised hosts has not been well understood until recently, and particularly, data concerning risk factors for LRTI in transplant recipients are still limited.3-5,27-29 Our institutions have been consistently utilizing multiplex PCR for clinically relevant respiratory viruses for over a decade, allowing us to identify risk factors for HCoV LRTI in the current PCR era. Multivariable logistic regression analyses revealed additional risk factors, relatively less commonly appreciated as risk factors for LRTI due to other respiratory viruses in HCT recipients.7,13,15,16,30,31 Whether these factors are also relevant to LRTI due to SARS-CoV-2 in this high risk population requires further study, although some studies have already shown male sex as a risk factor for poor outcomes related to SARS-CoV-2 in cancer patients.32,33 The presence of respiratory copathogens has not been well recognized as a risk factor for progression to LRTI due to HCoV or other respiratory viruses perhaps other than parainfluenza virus in HCT recipients.4,5,7,13,29,31,34-36 It is possible that some previous studies either did not evaluate this as a potential risk factor, or did not have the diagnostic capability to assess for this in the pre-PCR era. Overall, progression rates in patients with HCoV URTI are lower than those with other respiratory viruses, which may indicate that having more virulent respiratory viral copathogens (eg, RSV, human metapneumovirus) increases the risk of progression to LRTI.13,37,38 Revisiting the role of respiratory copathogens in LRTI outcomes is worth considering in this current molecular diagnostic era.
Hypoalbuminemia was associated with occurrence of HCoV LRTI, but not with progression from URTI to LRTI. Eichenberger et al also reported hypoalbuminemia was associated with occurrence of LRTI in logistic regression models in HCT recipients.5 Among our 43 patients with HCoV LRTI, 18 presented with HCoV LRTI, who were included into logistic regression models, but not into Cox regression models. This may partly explain the difference in association with outcomes and implies that hypoalbuminemia is a marker of severe illness due to LRTI rather than a risk factor for progression from URTI to LRTI. A similar observation was found for ISI, although the caveat is that our sample size only allowed us to use this scoring index as a continuous variable in multivariable models.15 Assessments of risk factors for progression from URTI to LRTI are particularly relevant for the design of early-intervention strategies to reduce the risk of disease progression. Further studies are needed to assess whether ISI as a discrete variable can predict progression to HCoV LRTI.
Important negative findings in the present study include that obesity and higher viral loads were not associated with increased risk of either occurrence of HCoV LRTI or progression to LRTI. Both factors have been identified as risk factors for progressive disease in SARS-CoV-2 infection.39-43 However, some studies in immunocompromised hosts did not demonstrate the association between obesity and poor outcomes with SARS-CoV-2 infection.6,32 Similarly, not all studies have been able to demonstrate a correlation of viral loads with outcome after SARS-CoV-2 infection,44,45 and previous studies for other respiratory viruses in transplant recipients also did not demonstrate that higher viral loads are a risk factor for progression to LRTI.7,13,46
Cytopenia in ≥1 cell line has been well recognized as a risk factor for LRTI due to majority of other clinically relevant respiratory viruses in immunocompromised hosts.7,13,31,34,35 A recent paper has shown an association with HCoV LRTI outcome, but another has not.4,5 In our analysis, cytopenias were significant when analyzed in univariable models and as part of the ISI but did not reach statistical significance in adjusted models when analyzed individually (Figure 1; supplemental Table 1). Our study also differs from other studies in terms of overall rates of LRTI.4,5,27 This could be due to a differential distribution of risk factors for LRTI as well as less complete virologic documentation of mild URTI.
The current study showed that patients with proven/probable HCoV LRTI had worse overall survival compared with those with possible LRTI. The mortality rates in patients with proven/probable HCoV LRTI were high regardless of the presence of respiratory copathogens, especially with oxygen requirement at the time of LRTI diagnosis. Overall, these results are consistent with previous studies of other respiratory viruses, suggesting the significance of virologic documentation of lower respiratory tract involvement by BAL.12,47,48 Additional studies are needed to assess whether proven/probable LRTI is an independent risk factor for mortality or whether the BAL procedure is just a marker of severe illness (eg, sicker patients are more likely to undergo BAL procedures).
This study has several limitations. Although this cohort represents one of the largest number of allogeneic HCT recipients infected with HCoV, the sample size was nonetheless too small to perform full multivariable Cox models to assess the independent effect of hyperglycemia on progression from URTI to LRTI from steroid dose. HbA1c may be a better indicator to predict LRTI outcome than glucose level given that HbA1c has less variability within subjects. No significant association between HbA1c and LRTI outcome was found in univariable analyses (supplemental Table 1); however, a substantial proportion of subjects did not have an HbA1c level within 3 months before HCoV diagnosis, and further studies are needed to address this question. Proven/probable LRTI may be more relevant outcomes given different mortality rates seen between patients with proven/probable LRTI and those with possible LRTI. However, our sample size did not allow us to analyze for these outcomes separately. At our institutions, HCT recipients with lower respiratory tract symptoms and radiographic abnormalities typically undergo BAL procedures. Nevertheless, the ultimate decision was deferred to the attending physicians, and some proven LRTI might have been classified as possible LRTI. Since many institutions do not routinely perform BAL for virologic confirmation, our results for any LRTI outcomes, from a practical point of view, can be broadly applicable. Many studies of respiratory viruses in HCT recipients have used definitions of LRTI that include possible cases.15,49-52 Lastly, given the nature of retrospective studies, we cannot rule out the possibility of other confounders.
In conclusion, our risk factor analyses for HCoV LRTI outcomes demonstrated unique features for HCoV compared with other respiratory viruses previously evaluated in HCT recipients. Whether these observations are also applicable to SARS-CoV-2 in HCT recipients requires further study. Assessing the independent effect of hyperglycemia from the use of steroids on progression from URTI to LRTI due to HCoV or other respiratory viruses is warranted, as this might provide an opportunity for interventions, including glycemic control, to reduce the risk of LRTI for this vulnerable population.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Ashley Akramoff and Anthony Mallory for data collection and Chris Davis for database services.
This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases, grant K23AI139385 (C.O.), National Heart, Lung, and Blood Institute grants R01HL081595 and K24HL093294 (M.B.), and National Cancer Institute grants CA18029 (W.M.L. [clinical database]) and grant CA15704 (H.X.); the Fred Hutchinson Cancer Research Center Vaccine and Infectious Disease Division (biorepository); and a Seattle Children’s Research Institute Clinical Research Scholars Program Award (C.O.).
Footnotes
Original data are available by request to the corresponding author (cogimi@fredhutch.org).
Authorship
Contribution: C.O. designed this study, assisted in analysis, interpreted results, and wrote the manuscript; H.X. and W.M.L. performed the statistical analysis and wrote the manuscript; A.W., M.U.O., and K.K.M. provided clinical input, interpreted results, and reviewed the manuscript; K.R.J. provided technical oversight for laboratory and reviewed the manuscript; and J.A.E. and M.B. provided oversight, designed the study, interpreted results, and reviewed the manuscript.
Conflict-of-interest disclosure: A.W. reports research support from Ansun Biopharma, Allovir, VB Tech, and Amazon and is an advisory board member for Kyorin Pharmaceutical. J.A.E. reports research support from AstraZeneca, Merck, Pfizer, GlaxoSmithKline, and Novavax; is an advisory board member and consultant for Sanofi Pasteur; and is a consultant for Meissa Vaccines. M.B. reports research support from Amazon, GSK, Regeneron, Ridgeback, Janssen, Gilead, and VirBio; consultant fees from Allovir, Janssen, Gilead, Moderna, and VirBio; and is an advisory board member for Evrys Bio (option to purchase shares). Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. The remaining authors declare no competing financial interests.
Correspondence: Chikara Ogimi, Pediatric Infectious Diseases Division, Seattle Children’s Hospital, 4800 Sand Point Way NE, MA.7.226, Seattle, WA 98105; e-mail: cogimi@fredhutch.org.
References
- 1.ElGohary GM, Hashmi S, Styczynski J, et al. The risk and prognosis of COVID-19 infection in cancer patients: a systematic review and meta-analysis [published online ahead of print 30 July 2020]. Hematol Oncol Stem Cell Ther. 10.1016/j.hemonc.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg SB. Update on human rhinovirus and coronavirus infections. Semin Respir Crit Care Med. 2016;37(4):555-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogimi C, Waghmare AA, Kuypers JM, et al. Clinical significance of human coronavirus in bronchoalveolar lavage samples from hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2017;64(11):1532-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piñana JL, Xhaard A, Tridello G, et al. ; Infectious Diseases Working Party of the European Society for Blood and Marrow Transplantation and Infectious Complications Subcommittee of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH) . Seasonal human coronaviruses respiratory tract infection in recipients of allogeneic hematopoietic stem cell transplantation [published online ahead of print 29 August 2020]. J Infect Dis. 10.1093/infdis/jiaa553 [DOI] [Google Scholar]
- 5.Eichenberger EM, Soave R, Zappetti D, et al. Incidence, significance, and persistence of human coronavirus infection in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2019;54(7):1058-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah GL, DeWolf S, Lee YJ, et al. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J Clin Invest. 2020;130(12):6656-6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo S, Gooley TA, Kuypers JM, et al. Human metapneumovirus infections following hematopoietic cell transplantation: factors associated with disease progression. Clin Infect Dis. 2016;63(2):178-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell AP, Guthrie KA, Englund JA, et al. Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant. Clin Infect Dis. 2015;61(2):192-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44(7):2382-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuypers J, Martin ET, Heugel J, Wright N, Morrow R, Englund JA. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics. 2007;119(1):e70-e76. [DOI] [PubMed] [Google Scholar]
- 11.Xu M, Qin X, Astion ML, et al. Implementation of filmarray respiratory viral panel in a core laboratory improves testing turnaround time and patient care. Am J Clin Pathol. 2013;139(1):118-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo S, Xie H, Campbell AP, et al. Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis. 2014;58(10):1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waghmare A, Xie H, Kuypers J, et al. Human rhinovirus infections in hematopoietic cell transplant recipients: risk score for progression to lower respiratory tract infection. Biol Blood Marrow Transplant. 2019;25(5):1011-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammer MJ, Casper C, Gooley TA, O’Donnell PV, Boeckh M, Hirsch IB. The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2009;15(3):344-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah DP, Ghantoji SS, Ariza-Heredia EJ, et al. Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood. 2014;123(21):3263-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waghmare A, Englund JA, Boeckh M. How I treat respiratory viral infections in the setting of intensive chemotherapy or hematopoietic cell transplantation. Blood. 2016;127(22):2682-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung SH, Jang HC, Lee SS, et al. The impact of hyperglycemia on risk of severe infections during early period of induction therapy in patients with newly diagnosed multiple myeloma. BioMed Res Int. 2014;2014:413149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sopfe J, Pyle L, Keating AK, et al. Malglycemia is associated with poor outcomes in pediatric and adolescent hematopoietic stem cell transplant patients. Blood Adv. 2019;3(3):350-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai Y, Shi S, Yang F, et al. Fasting blood glucose level is a predictor of mortality in patients with COVID-19 independent of diabetes history. Diabetes Res Clin Pract. 2020;169:108437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyal A, Gupta S, Gupta Y, Tandon N. Proposed guidelines for screening of hyperglycemia in patients hospitalized with COVID-19 in low resource settings. Diabetes Metab Syndr. 2020;14(5):753-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh AK, Singh R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res Clin Pract. 2020;167:108382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hulme KD, Gallo LA, Short KR. Influenza virus and glycemic variability in diabetes: a killer combination? Front Microbiol. 2017;8:861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reading PC, Allison J, Crouch EC, Anders EM. Increased susceptibility of diabetic mice to influenza virus infection: compromise of collectin-mediated host defense of the lung by glucose? J Virol. 1998;72(8):6884-6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiu F, Stanojcic M, Diao L, Jeschke MG. Stress hyperglycemia, insulin treatment, and innate immune cells. Int J Endocrinol. 2014;2014:486403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijsman CA, Mooijaart SP, Westendorp RG, Maier AB. Responsiveness of the innate immune system and glucose concentrations in the oldest old. Age (Dordr). 2012;34(4):983-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, Fang P, He R, et al. O-GlcNAc transferase promotes influenza A virus-induced cytokine storm by targeting interferon regulatory factor-5. Sci Adv. 2020;6(16):eaaz7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piñana JL, Madrid S, Pérez A, et al. Epidemiologic and clinical characteristics of coronavirus and bocavirus respiratory infections after allogeneic stem cell transplantation: a prospective single-center study. Biol Blood Marrow Transplant. 2018;24(3):563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hakki M, Rattray RM, Press RD. The clinical impact of coronavirus infection in patients with hematologic malignancies and hematopoietic stem cell transplant recipients. J Clin Virol. 2015;68:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pochon C, Voigt S. Respiratory virus infections in hematopoietic cell transplant recipients. Front Microbiol. 2019;9:3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;59(Suppl 5):S344-S351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah DP, Shah PK, Azzi JM, Chemaly RF. Parainfluenza virus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: a systematic review. Cancer Lett. 2016;370(2):358-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuderer NM, Choueiri TK, Shah DP, et al. ; COVID-19 and Cancer Consortium . Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khawaja F, Chemaly RF. Respiratory syncytial virus in hematopoietic cell transplant recipients and patients with hematologic malignancies. Haematologica. 2019;104(7):1322-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kmeid J, Vanichanan J, Shah DP, et al. Outcomes of influenza infections in hematopoietic cell transplant recipients: application of an immunodeficiency scoring index. Biol Blood Marrow Transplant. 2016;22(3):542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dadwal S, Kriengkauykiat J, Cooper F, Tegtmeier B, Forman S, Ito J. Comparison of clinical features and outcomes in haematopoietic cell transplant recipients infected with 2009 pandemic H1N1 influenza A and seasonal influenza A virus. Bone Marrow Transplant. 2011;46:S26-S27. [Google Scholar]
- 37.Renaud C, Englund JA. Antiviral therapy of respiratory viruses in haematopoietic stem cell transplant recipients. Antivir Ther. 2012;17(1 Pt B):175-191. [DOI] [PubMed] [Google Scholar]
- 38.Ogimi C, Englund JA, Bradford MC, Qin X, Boeckh M, Waghmare A. Characteristics and outcomes of coronavirus infection in children: the role of viral factors and an immunocompromised state. J Pediatric Infect Dis Soc. 2019;8(1):21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westblade LF, Brar G, Pinheiro LC, et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell. 2020;38(5):661-671.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benotmane I, Gautier-Vargas G, Wendling MJ, et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am J Transplant. 2020;20(11):3162-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pujadas E, Chaudhry F, McBride R, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med. 2020;8(9):e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wadman M. Why obesity worsens COVID-19. Science. 2020;369(6509):1280-1281. [DOI] [PubMed] [Google Scholar]
- 43.Sales-Peres SHC, de Azevedo-Silva LJ, Bonato RCS, Sales-Peres MC, Pinto ACDS, Santiago Junior JF. Coronavirus (SARS-CoV-2) and the risk of obesity for critically illness and ICU admitted: Meta-analysis of the epidemiological evidence. Obes Res Clin Pract. 2020;14(5):389-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Argyropoulos KV, Serrano A, Hu J, et al. Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with outcome and symptoms. Am J Pathol. 2020;190(9):1881-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Pina JM, Rodríguez Rodriguez M, Castro Quismondo N, et al. Clinical course and risk factors for mortality from COVID-19 in patients with haematological malignancies. Eur J Haematol. 2020;105(5):597-607. [DOI] [PubMed] [Google Scholar]
- 46.Seo S, Xie H, Leisenring WM, et al. Risk factors for parainfluenza virus lower respiratory tract disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(1):163-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo S, Waghmare A, Scott EM, et al. Human rhinovirus detection in the lower respiratory tract of hematopoietic cell transplant recipients: association with mortality. Haematologica. 2017;102(6):1120-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vakil E, Sheshadri A, Faiz SA, et al. Risk factors for mortality after respiratory syncytial virus lower respiratory tract infection in adults with hematologic malignancies. Transpl Infect Dis. 2018;20(6):e12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi SM, Boudreault AA, Xie H, Englund JA, Corey L, Boeckh M. Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood. 2011;117(19):5050-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisher BT, Danziger-Isakov L, Sweet LR, et al. A multicenter consortium to define the epidemiology and outcomes of inpatient respiratory viral infections in pediatric hematopoietic stem cell transplant recipients. J Pediatric Infect Dis Soc. 2018;7(4):275-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sim SA, Leung VKY, Ritchie D, Slavin MA, Sullivan SG, Teh BW. Viral respiratory tract infections in allogeneic hematopoietic stem cell transplantation recipients in the era of molecular testing. Biol Blood Marrow Transplant. 2018;24(7):1490-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah DP, Ghantoji SS, Shah JN, et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013;68(8):1872-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.