Abstract
Aims/Introduction
Several clinical trials reported the effects of sodium–glucose cotransporter (SGLT) inhibitors in type 1 diabetes patients. This meta‐analysis aimed to assess the efficacy and safety of SGLT inhibitors in type 1 diabetes patients.
Materials and Methods
Relevant studies were identified in the PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure and Wan Fang databases through 1 April 2020. Differences were expressed as the 95% confidence interval (CI) or weighted mean difference (WMD) for continuous outcomes, and risk ratio (RR) for discontinuous outcomes.
Results
A total of 13 RCTs with 7,962 cases were included. SGLT inhibitors reduced the fasting plasma glucose level (WMD −1.320 mmol/L, 95% CI −1.609 to −1.031, P < 0.001), glycated hemoglobin level (WMD −0.386%, 95% CI −0.431 to −0.342, P < 0.001) and daily total insulin dose (WMD −5.403, 95% CI −7.218 to −3.859, P < 0.001). However, higher risks of diabetic ketoacidosis (RR 5.042, 95% CI 3.160–8.046, P < 0.001), urinary tract infections (RR 1.259, 95% CI 1.034–1.533,P = 0.022) and genital infections (RR 2.995, 95% CI 1.953–4.594, P < 0.001) were associated with SGLT inhibitors, but SGLT inhibitors did not increase the hypoglycemia risk (RR 0.980, 95% CI 0.840–1.144,P = 0.799). In subgroup analysis, with a significant reduction of fasting plasma glucose, glycated hemoglobin and daily insulin doses, SGLT1/2 inhibitor did not increase genitourinary tract infections compared with a placebo.
Conclusions
SGLT2 and SGLT1/2 inhibitors can improve glycemic control in patients with type 1 diabetes.
Keywords: Diabetes mellitus type 1, Effects, Sodium–glucose cotransporter inhibitors
The present meta‐analysis analyzed the efficacy and safety of sodium–glucose co‐transporter (SGLT) 2 inhibitors and dual SGLT1/2 inhibitors added to insulin for patients with type 1 diabetes, and carried out a subgroup analysis between SGLT2 inhibitors and dual SGLT1/2 inhibitors. Our results showed that SGLT inhibitors as add‐on therapy is associated with improved glycemic control, but increased risk for urinary tract infections and diabetic ketoacidosis, proving the potential benefits of SGLT inhibitors with careful use for type 1 diabetes patients.

Introduction
Type 1 diabetes is caused by organ‐specific autoimmune destruction of insulin‐producing β‐cells in the pancreatic islets of Langerhans, thereby leading to insulin deficiency and hyperglycemia 1 . Chronic diabetic complications, such as macrovascular and microvascular diseases, are the leading causes of mortality and disability in type 1 diabetes patients 2 , 3 . Type 1 diabetes patients are also at an increased risk of diabetic ketoacidosis (DKA), a serious condition caused by an absolute or a relative deficiency of circulating insulin levels, commonly due to precipitating factors, such as intercurrent illness or interruption of insulin therapy 4 .
Intensive insulin treatment has been shown to reduce the onset and/or progression of microvascular and macrovascular complications in patients with type 1 diabetes 5 , 6 ; however, even with the development of rapid‐ and long‐acting insulin analogs, and improvements in insulin delivery devices, 75% of adults with type 1 diabetes fail to achieve the target glycated hemoglobin (HbA1c) level of <7.0% (<53 mmol/mol) recommended by the American Diabetes Association 7 , 8 , and many suffer from significant hypoglycemic episodes. On average, patients with type 1 diabetes suffer from >40 episodes of hypoglycemia per year, and more specifically, 14–20% of patients aged >50 years were shown to have severe hypoglycemic events 7 , 9 , 10 , 11 , 12 , 13 . In addition to these issues, these patients suffer from weight gain, which increases the difficulty of achieving their therapeutic targets. Due to these therapeutic limitations, the addition of a second therapeutic agent with an insulin‐independent mechanism of action might allow patients with type 1 diabetes to improve their glycemic control without increasing the insulin dose and to possibly even reduce the insulin dose. Although none of these agents can replace insulin therapy, some enable easier management of diabetes.
Sodium–glucose cotransporters (SGLTs) are proteins within the kidney that play a key role in the reabsorption of glucose. SGLT2 is the major glucose transporter, and is responsible for 90% of the reabsorption of glucose, whereas SGLT1, another glucose transporter, is responsible for the remaining 10% of glucose reabsorption 14 , 15 , 16 , 17 . Thus, inhibition of SGLT prevents glucose from being reabsorbed into the bloodstream, thereby decreasing blood glucose levels. SGLT2 inhibitors have been reported to constitute a new class of antidiabetic agents that have been shown to improve glycemic control and reduce bodyweight with little risk of hypoglycemia in patients with type 2 diabetes 18 , 19 . Therefore, we investigated whether SGLT inhibitors benefit patients with type 1 diabetes.
The present meta‐analysis was carried out to evaluate the efficacy and safety of SGLT inhibitors in patients with type 1 diabetes.
Methods
Search strategy
A literature search was carried out in the PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure and Wan Fang databases from inception to 1 April 2020. We searched medical subject headings (MeSH) terms with entry terms. For example, for the PubMed database, we searched (“Diabetes Mellitus, Type 1”[MeSH] OR type 1 diabetes OR type 1 diabetes mellitus OR T1DM OR Diabetes Mellitus, Insulin‐Dependent OR Diabetes Mellitus, Insulin Dependent OR Insulin‐Dependent Diabetes Mellitus) AND (“Sodium‐Glucose Transporter 1”[MeSH] OR “Sodium‐Glucose Transporter 2”[MeSH] OR “Sodium‐Glucose Transport Proteins”[MeSH] OR Sodium‐glucose cotransporter OR SGLT OR SGLT1 OR SGLT2 OR Dapagliflozin OR Canagliflozin OR Empagliflozin OR Ertugliflozin OR Ipragliflozin OR Luseogliflozin OR Tofogliflozin OR Sotagliflozin OR Gliflozins). Eligible randomized trials fulfilled the following criteria: (i) a randomized controlled clinical trial assessing the efficacy and safety of SGLT inhibitors versus a placebo; (ii) studies with data types providing categorization and continuity data; (iii) articles written in English or Chinese; and (iv) studies involving human subjects. Articles were excluded if they were reviews, if the design did not involve the correct controls or if the study had no outcome. We tried to contact the authors for missing data when necessary.
Study selection, quality assessment and risk of bias
The results of the systematic searches were imported to a reference manager, Endnote X9 (Clarivate Analytics, Philadelphia, PA, USA) software, to remove duplicates. Then, two reviewers (HL Zou and LL Liu) independently screened titles, abstracts and full‐text articles for inclusion; if any disagreements regarding whether a study should be included arose, the two researchers discussed it first, and disparities were settled by a third reviewer (Y Xiao). We included English‐language studies of patients with type 1 diabetes who received SGLT inhibitor treatments. SGLT inhibitors included dapagliflozin, canagliflozin, empagliflozin, ipragliflozin and sotagliflozin. We included randomized controlled trials (RCTs) evaluating efficacy outcomes (fasting plasma glucose [FPG], HbA1c, the mean amplitude of glucose excursion [MAGE] and insulin dose) and safety outcomes (genital mycotic infection, urinary tract infections, hypoglycemia, DKA and diarrhea). We also extracted information on the general study, and participant characteristics, interventions, comparisons and outcome results. A second reviewer confirmed the abstracted data by using standardized forms.
Two reviewers (HL Zou and J Guo) evaluated the eligibility of each study, extracted the data and determined the risk of bias. In addition, any controversies were resolved through consensus; if any further ambiguity remained, it was discussed with a third person (ZG Zhou).
Variables
We extracted the efficacy outcomes and safety outcomes. Considering the definitions across studies, a total of six efficacy parameters, namely, HbA1c level (%), FPG level (mmol/L), MAGE (mg/dL), basal insulin dose (IU/day), bolus insulin dose (IU/day) and total insulin dose (IU/day) were extracted. Safety was assessed in terms of five types of adverse events, including genital mycotic infections, urinary tract infections, hypoglycemia, DKA and diarrhea.
Statistical analysis
We created a set of detailed evidence tables. We carried out a meta‐analysis only when data were sufficient (from at least two trials) and studies were sufficiently homogenous with respect to key variables (population characteristics, study duration and medication dosing). For continuous outcomes, we extracted the weighted mean differences (WMDs) and 95% confidence intervals (95% CIs) from baseline for both the experimental group and placebo groups. For dichotomous outcomes, we calculated the risk ratios (RRs) and 95% CIs. We carried out subgroup analyses based on SGLT 2 inhibitors and dual SGLT1/2 inhibitors. We established subgroups stratified by body mass index (BMI) 20 and age 21 . We also focused on the incidence of DKA without hyperglycemia between the two groups. All the parameters measured in the subgroup analyses were the same as those aforementioned.
We evaluated the quality of the articles using the Jadad scale. The data were analyzed with Stata V.12.0 software (StataCorp, College Station, TX, USA). The heterogeneity of the studies was assessed by the Q test and the I 2 statistic 22 , and the test level was set at 0.05. If the heterogeneity test results were P > 0.05 and I 2 < 50%, the combined effect was calculated with a fixed effects model; otherwise, the random effects model was used. Possible publication bias was assessed with funnel plots and Egger’s test. The clipping method was used if publication bias existed. For articles with evidence of heterogeneity, we ensured the stability of results by carrying out a sensitivity analysis. Literature subtraction was used if sensitivity analysis found the source of heterogeneity, otherwise the random effects model was used.
Results
As shown in the flowchart (Figure 1), after excluding duplicates, a total of 2,330 records were identified, 2,185 of which were excluded, because they had irrelevant titles and abstracts. A total of 145 publications underwent further evaluation to eliminate those that did not meet the inclusion criteria. Then, 132 articles were removed, because they were reviews, case reports or other studies that were not RCTs, were not written in English or Chinese, or lacked eligible populations, sufficient data or the outcomes of interest. After exclusion, 13 studies (7,962 participants) satisfied the inclusion criteria for the meta‐analysis. Among these 13 articles, two studies by Dandona et al. carried out in 2017 23 and 2018 24 reported results from different stages of the Efficacy and Safety of Dapagliflozin in Patients with Inadequately Controlled Type 1 Diabetes (DEPICT‐1) study (NCT02268214). All included RCTs compared SGLT inhibitors with a placebo under the condition of background insulin treatment. Dapagliflozin was investigated in four studies 23 , 24 , 25 , 26 , canagliflozin was investigated in one study 27 , empagliflozin was investigated in two studies 28 , 29 , ipragliflozin was investigated in one study 30 and sotagliflozin was investigated in five studies 31 , 32 , 33 , 34 , 35 .
Figure 1.
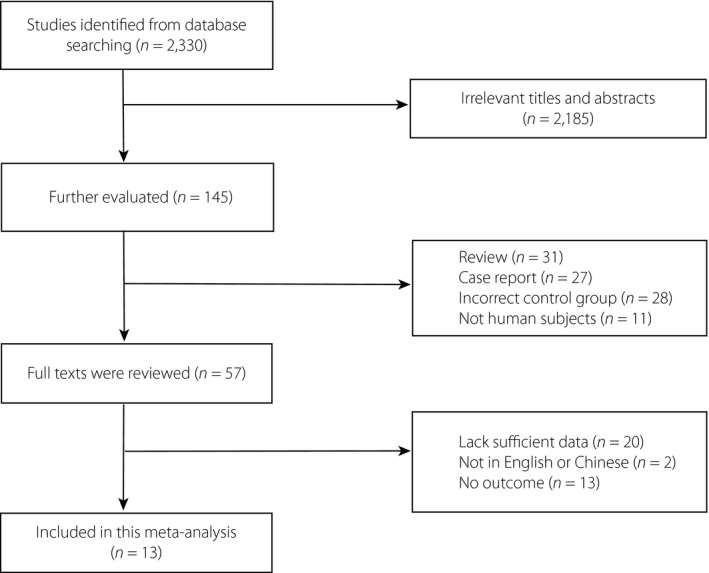
Flow diagram of studies identified, included and excluded.
The detailed characteristics (Table 1) and quality assessment (Table S1) of the included studies are shown. Two reviewers, following an evidence grading scheme recommended in the Agency for Healthcare Research and Quality guide for carrying out comparative effectiveness reviews 36 , sequentially graded the studies’ limitations, consistency, directness, precision and potential reporting bias for the evidence for each outcome and comparison. All studies followed the principles of randomization and blinding. Risk of bias assessment showed that most studies had a low risk.
Table 1.
Baseline characteristics of trials included in the meta‐analysis
| Article | NCT ID | Year | Intervention | Follow‐up week | Participants, n (E/C) | Female (n) | Mean age (years) | Mean diabetes duration (years) | Mean BMI (kg/m2) | Mean HbA1c (%) | SBP ≥130 mmHg (%) | Overweight or obesity(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sands et al. 31 | NCT01742208 | 2015 | Sotagliflozin (400 mg) | 4 | 33 (16/17) | 17 | 39.1 | 20.3 | 26.6 | 7.96 | NR | NR |
| Garg et al. 32 | NCT02531035 | 2017 | Sotagloflizin (400 mg) | 24 | 1,402 (699/703) | 705 | 42.9 | 20.1 | 28.2 | 8.23 | 29.0 | 71.0 † |
| Danne et al. 33 | NCT02421510 | 2018 | Sotagliflozin (200 mg/400 mg) | 52 | 782 (524/258) | 376 | 41.2 | 18.4 | 27.8 | 7.75 | 32.1 | 29.9 ‡ |
| Buse et al. 34 | NCT02384941 | 2018 | Sotagliflozin (200 mg/400 mg) | 52 | 793 (525/268) | 410 | 46.1 | 24.4 | 29.7 | 7.57 | 23.2 | 44.0 ‡ |
| Baker et al. 35 | NCT02459899 | 2019 | Sotagliflozin (75 mg/200 mg/400 mg) | 12 | 141 (105/36) | 73 | 45.6 | 24.1 | 29.2 | 8.02 | 22.0 | 36.9 ‡ |
| Dandona et al. 23 | NCT02268214 | 2017 | Dapagliflozin (5 mg/10 mg) | 24 | 833 (573/260) | 405 | 42.5 | 20.3 | 28.3 | 8.53 | 14.6 § | NR |
| Dandona et al. 24 | 2018 | Dapagliflozin (5 mg/10 mg) | 52 | 833 (573/260) | 405 | 42.5 | 20.3 | 28.3 | 8.53 | NR | ||
| Mathieu et al. 26 | NCT02460978 | 2018 | Dapagliflozin (5 mg/10 mg) | 24 | 813 (541/272) | 455 | 42.7 | 19.3 | 27.6 | 8.44 | NR | NR |
| Kuhadiya et al. 25 | NCT02518945 | 2016 | Dapagliflozin (10 mg) | 12 | 26 (17/9) | 16 | 54.9 | 27.1 | 29.6 | 7.66 | NR | NR |
| Henry et al. 27 | NCT02139943 | 2015 | Canagliflozin (100 mg/300 mg) | 18 | 351 (234/117) | 154 | 42.3 | 22.4 | 28.1 | 7.90 | NR | NR |
| Pieber et al. 28 | NCT01969747 | 2015 | Empagliflozin (2.5 mg/10 mg/25 mg) | 4 | 75 (56/19) | 22 | 41.0 | 20.1 | 25.7 | 8.24 | NR | NR |
| Rosenstock et al. 29 | NCT02414958 NCT02580591 (The EASE Program) | 2018 | Empagliflozin (2.5 mg/10 mg/25 mg) | 52 | 1,705 (1,221/484) | 877 | 44.0 | 21.7 | 28.6 | 8.14 | NR | NR |
| Kaku et al. 30 | NCT02897219 | 2019 | Ipragliflozin (50 mg) | 24 | 175 (115/60) | 93 | 49.2 | NR | 24.5 | 8.68 | NR | 40.0 † |
BMI, body mass index; E/C, number of patient treated by sodium–glucose cotransporter inhibitors/number of patient treated by a placebo; HbA1c, glycated hemoglobin; NCT ID, National Clinical Trial number; NR, not reported; SBP, systolic blood pressure.
Percentage of patients with body mass index (BMI) ≥25.
Percentage of patients with BMI ≥30.
Percentage of patients with hypertension.
Meta‐analysis
The baseline characteristics extracted from each study are presented in Table 1. All the type 1 diabetes patients included in the 13 studies were aged >18 years and received optimal insulin treatment without a history of severe adverse events. In those studies, participants were randomly divided into an experimental group and a control group. In addition to insulin treatment, SGLT inhibitors were used in the experimental group. The efficacy and safety indicators were compared between the two groups.
Glycemic efficacy outcomes
FPG
We observed no heterogeneity among the five studies evaluating FPG in the meta‐analysis, indicating a consistent drug effect. The meta‐analysis using a fixed effects model showed that SGLT inhibitors markedly reduced the FPG level compared with the placebo (WMD −1.320, 95% CI −1.609 to −1.031, P < 0.001, I 2 = 0.0%, five comparisons, 1,711 participants; Figure 2a). In the subgroup analysis of different drug type, both SGLT2 inhibitors (three comparisons, 276 participants) and the dual SGLT1/2 inhibitor (two comparisons, 1,435 participants) were associated with a lower FPG level (SGLT2 inhibitors WMD −1.420, 95% CI −2.384 to −0.457, P = 0.004, I 2 = 34.8%; dual SGLT1/2 inhibitor WMD −1.310, 95% CI −1.613 to −1.008, P < 0.001, I 2 = 0.0%; Figure 2b). BMI and age did not influence the effect of SGLT inhibitors on FPG (Figure S1a,b).
Figure 2.
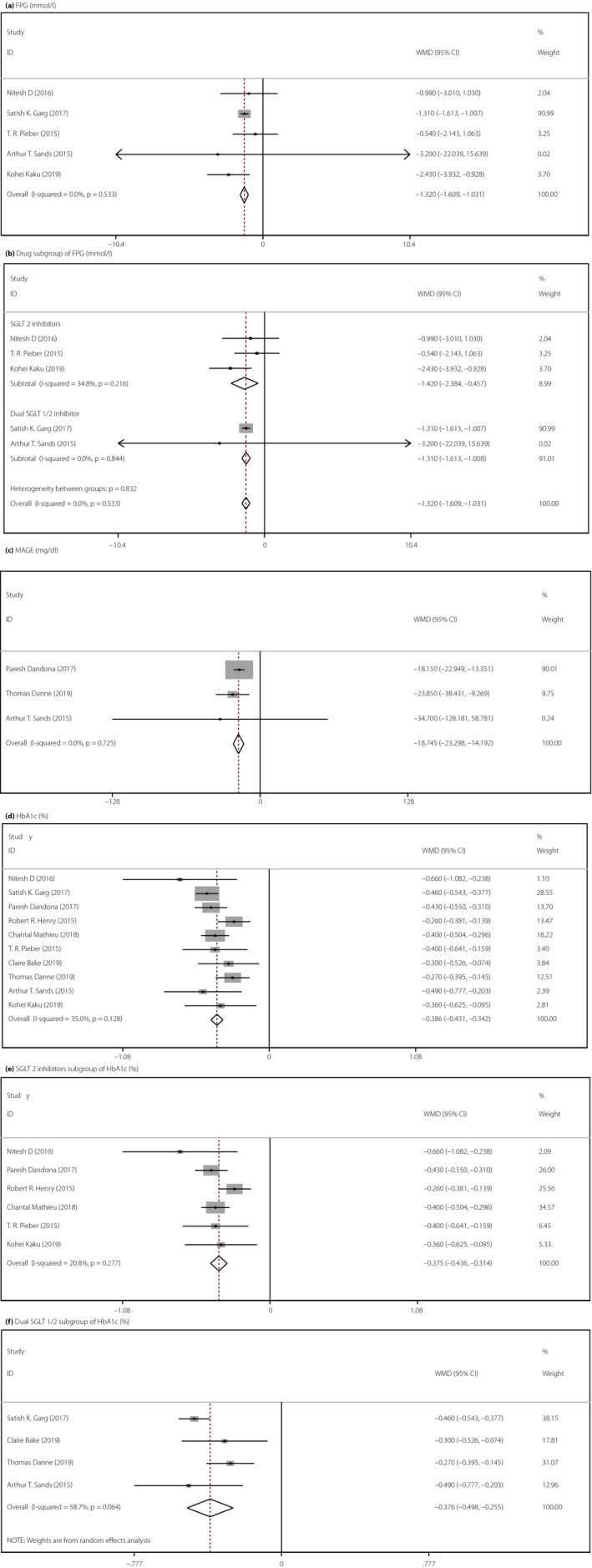
Forest illustration of meta‐analysis comparing the efficacy of add‐on sodium–glucose cotransporter (SGLT) inhibitor therapy for type 1 diabetes. (a) Fasting plasma glucose (FPG; mmol/L), χ2 = 3.15, I 2 = 0.0%, P = 0.533, weighted mean difference (WMD) = −1.320, 95% confidence interval (CI) −1.609 to −1.031, z = 8.96, P < 0.001. (b) Drug subgroup of fasting plasma (mmol/L), SGLT2: χ2 = 3.07, I 2 = 34.8%, P = 0.216, WMD = −1.420, 95% CI −2.384 to −0.457, z = 2.89, P = 0.004; dual SGLT1/2: χ2 = 0.04, I 2 = 0.0%, P = 0.844, WMD = −1.310, 95% CI −1.613 to −1.008, z = 8.48, P < 0.001. (c) Mean amplitude of glucose excursion (MAGE; mg/dL), χ2 = 0.64, I 2 = 0.0%, P = 0.725, WMD = −18.745, 95% CI −23.289 to −14.192, z = 8.07, P < 0.001. (d) Glycated hemoglobin (HbA1c; %), χ2 = 13.84, I 2 = 35.0%, P = 0.128, WMD = −0.386, 95% CI −0.431 to −0.342, z = 17.07, P < 0.001. (e) SGLT2 subgroup of HbA1c, χ2 = 6.32, I 2 = 20.8%, P = 0.277, WMD = −0.375, 95% CI −0.436 to −0.341, z = 12.04, P < 0.001. (f) Dual SGLT1/2 subgroup of HbA1c, χ2 = 7.27, I 2 = 58.7%, P = 0.064, WMD = −0.376, 95% CI −0.498 to −0.255, z = 6.07, P < 0.001.
MAGE
Three RCTs evaluated MAGE by continuous glucose monitoring. The heterogeneity test result showed no heterogeneity in the present meta‐analysis, suggesting a consistent drug effect. Our meta‐analysis using a fixed effects model showed that compared with placebo, SGLT inhibitors significantly improved the glycemic control of patients with type 1 diabetes (WMD −18.745, 95% CI −23.289 to −14.192, P < 0.001, I 2 = 0.0%, three comparisons, 1,648 patients; Figure 2c). We did not carry out a subgroup analysis, because just three studies evaluated the effects of SGLT inhibitors on the MAGE.
HbA1c
A total of 10 studies were included in the analysis of HbA1c. Given the low heterogeneity, we used the fixed effects model for analysis. Compared with the placebo, SGLT inhibitors significantly reduced the HbA1c levels of patients (WMD −0.386, 95% CI −0.431 to −0.342, P < 0.001, I 2 = 35.0%, 10 comparisons, 4,631 participants; Figure 2d). In the subgroup analysis of different drug types, we obtained the same result: the dual SGLT inhibitor contributed to a reduction in HbA1c level in type 1 diabetes patients (WMD −0.376, 95% CI −0.498 to −0.255, P < 0.001, I 2 = 58.7%), and the effect of SGLT2 inhibitors on HbA1c levels was consistent with that of the dual SGLT inhibitor (WMD −0.375, 95% CI −0.426 to −0.314, P < 0.001, I 2 = 20.8%; Figure 2e–f). BMI and age did not influence the effect of SGLT inhibitors on the HbA1c level (Figure S1c–e).
Daily total, bolus and basal insulin doses
Significant differences were identified in the daily total (WMD −5.403, 95% CI −7.218 to −3.859, P < 0.001, I 2 = 64.3%, five comparisons, 3,218 participants), bolus (WMD −2.633, 95% CI −3.448 to −1.819, P < 0.001, I 2 = 30.1%, five comparisons, 2,418 patients) and basal (WMD −2.812, 95% CI −3.327 to −2.297, P < 0.001, I 2 = 48.6%, five comparisons, 2,418 patients) insulin doses between the SGLT inhibitor group and the placebo group among patients with type 1 diabetes (Figure 3a–c). High heterogeneity was observed among the studies for the daily total insulin doses, and sensitivity analysis showed no obvious outliers; the results were stable (Figure S2a).
Figure 3.
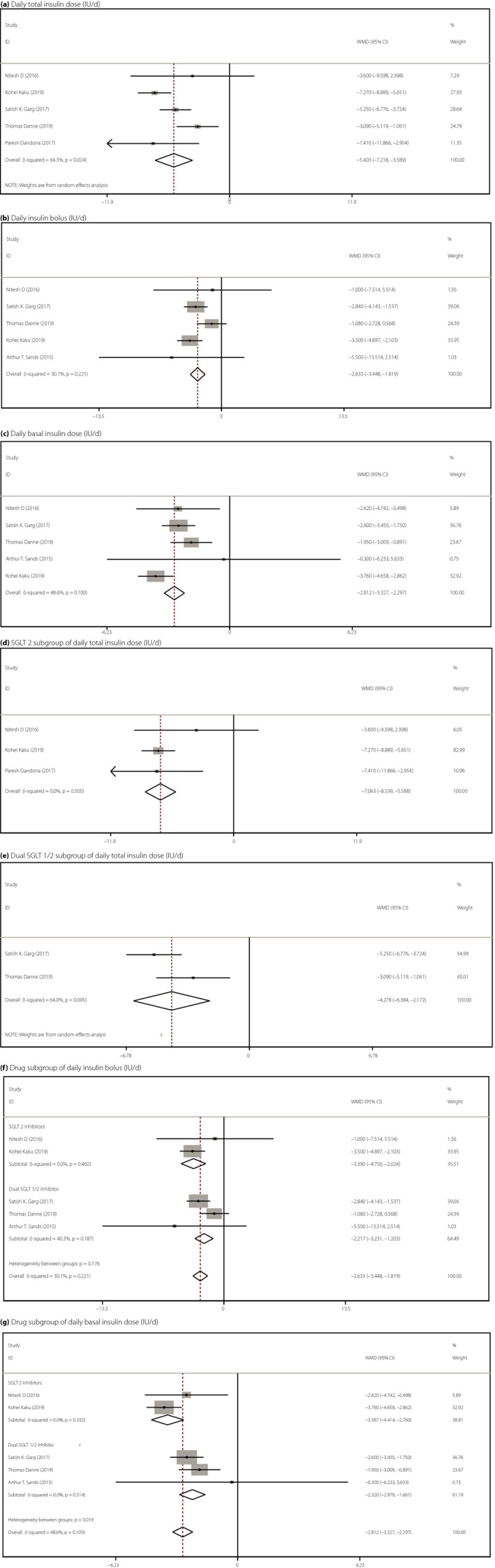
Forest illustration of meta‐analysis comparing the efficacy of add‐on sodium–glucose cotransporter (SGLT) inhibitor therapy on insulin dose for type 1 diabetes. (a) Daily total insulin dose (units per day), χ2 = 11.20, I 2 = 64.3%, P = 0.024, weighted mean difference (WMD) = −5.043, 95% confidence interval (CI) −7.218 to −3.859, z = 5.84, P < 0.001. (b) Daily insulin bolus (units per day), χ2 = 5.72, I 2 = 30.1%, P = 0.221, WMD = −2.633, 95% CI −3.448, −1.819, z = 6.34, P < 0.001. (c) Daily basal insulin dose (units per day), χ2 = 7.79, I 2 = 48.6%, P = 0.100, WMD = −2.812, 95% CI −3.327 to −2.297, z = 10.70, P < 0.001. (d) SGLT2 subgroup of total daily insulin dose (units per day), χ2 = 1.37, I 2 = 0.0%, P = 0.505, WMD = −7.063, 95% CI −8.539 to −5.588, z = 9.38, P < 0.001. (e) Dual SGLT1/2 subgroup of total daily insulin dose (units per day), χ2 = 2.78, I 2 = 64.0%, P = 0.095, WMD = −4.278, 95% CI −6.384 to −2.172, z = 3.98, P < 0.001. (f) Drug subgroup of daily insulin bolus (units per day), SGLT2: χ2 = 0.54, I 2 = 0.0%, P = 0.462, WMD = −3.390, 95% CI −4.756 to −2.024, z = 4.86, P < 0.001; dual SGLT1/2: χ2 = 3.35, I 2 = 40.3%, P = 0.187, WMD = −2.217, 95% CI −3.231 to −1.203, z = 4.29, P < 0.001. (g) Drug subgroup of basal insulin dose (units per day), SGLT2: χ2= 0.94, I 2 = 0.0%, P = 0.332, WMD = −3.587, 95% CI −4.414 to −2.760, z = 8.50, P < 0.001; dual SGLT1/2: χ2 = 1.33, I 2 = 0.0%, P = 0.514, WMD = −2.320, 95% CI −2.979 to −1.661, z = 6.90, P < 0.001.
Subgroup analyses by different drugs showed that the effects of the dual SGLT inhibitor (sotagliflozin) on the daily total insulin dose (WMD −4.728, 95% CI −6.384 to −2.172, P < 0.001, I 2 = 64.0%, two comparisons, 2,184 patients), daily insulin bolus (WMD −2.217, 95% CI −3.231 to −1.203, P < 0.001, I 2 = 40.3%, three comparisons, 2,217 patients) and basal insulin dose (WMD −2.320, 95% CI −2.979 to −1.661, P < 0.001, I 2 = 0.0%, three comparisons, 2,217 patients) were consistent with those of SGLT2 inhibitors (Figure 3d–f). BMI and age did not influence the effect of SGLT inhibitors on the insulin dose (Figure S3). Because high heterogeneity was observed in a subgroup of people with a BMI ≥28, an analysis was not carried out.
Safety outcomes
DKA
A total of 12 studies including 7,887 patients reported the risk of DKA. Given the low heterogeneity observed, the fixed effects model was used. Compared with a placebo, SGLT inhibitors were associated with a higher risk of DKA (RR 5.042, 95% CI 3.160–8.046, P < 0.001, I 2 = 0.0%, 12 comparisons, 7,887 participants; Figure 4a). Compared with a placebo, SGLT2 inhibitors increased the risk of DKA by 330% (RR 4.313, 95% CI 2.439–7.628, P = 0.001, I 2 = 0.0%, seven comparisons, 3,150 patients) and the dual SGLT1/2 inhibitor increased the risk of DKA by 580% (RR 6.825, 95% CI 2.979–15.635, P < 0.001, I 2 = 0.0%, five comparisons, 4,736 patients; Figure 4b). In BMI subgroups, we observed that SGLT inhibitors increased the risk of DKA by 964% in lean people (RR 10.638, 95% CI 2.496–45.331, P < 0.001, I 2 = 0.0%, four comparisons, 1,803 patients) and by 342% in obese people (RR 4.423, 95% CI 2.695–7.257, P < 0.001, I 2 = 0.0%, eight comparisons, 5,302 patients). Both older and younger populations had an increased risk of DKA after using SGLT inhibitors (Figure S4).
Figure 4.
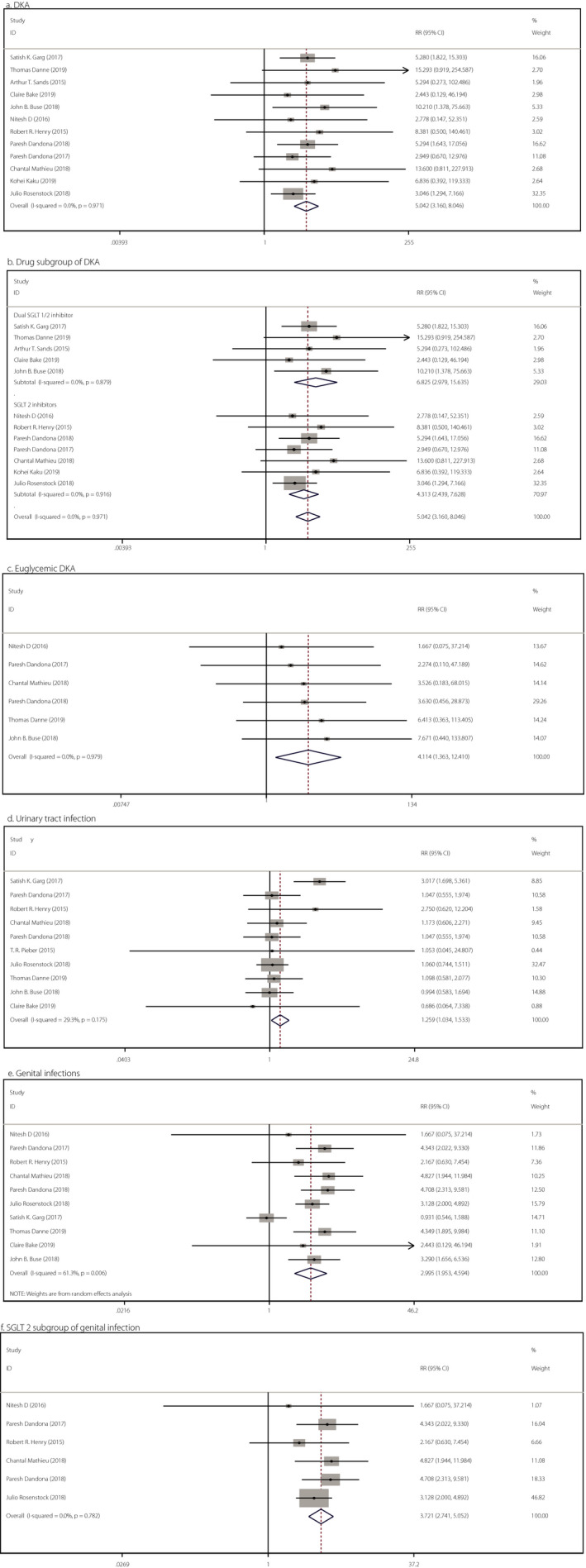
Forest illustration of meta‐analysis comparing the safety of add‐on sodium–glucose cotransporter (SGLT) inhibitor therapy for type 1 diabetes. (a) Diabetic ketoacidosis (DKA), χ2 = 3.96, I 2 = 0.0%, P = 0.971, RR = 5.042, 95% confidence interval (CI) 3.160–8.046, z = 6.792, P < 0.001. (b) Drug subgroup of DKA, dual SGLT1/2: χ2 = 1.19, I 2 = 0.0%, P = 0.879, RR = 6.825, 95% CI 2.979–15.635, z = 4.54, P < 0.001; SGLT2: χ2 = 2.04, I 2 = 0.0%, P = 0.916, RR = 4.313, 95% CI 2.439–7.628, z = 5.03, P < 0.001. (c) Euglycemic of DKA, χ2 = 0.77, I 2 = 0.0%, P = 0.979, RR = 4.114, 95% CI 1.363–12.410, z = 2.51, P = 0.012. (d) Urinary tract infections, χ2 = 12.73, I 2 = 29.3%, P = 0.175, RR = 1.259, 95% CI 1.034–1.533, z = 2.29, P = 0.022. (e) Genital infection, χ2 = 23.28, I 2 = 61.3%, P = 0.006, RR = 2.995, 95% CI 1.953–4.594, z = 5.03, P < 0.001. (f) SGLT2 subgroup of genital infection, χ2 = 2.46, I 2 = 0.0%, P = 0.782, RR = 3.721, 95% CI 2.741–5.052, z = 8.42, P < 0.001.
Six studies reported the incidence of DKA without hyperglycemia, and the meta‐analysis showed that SGLT inhibitors were associated with higher euglycemic DKA (RR 4.114, 95% CI 1.363–12.410, P = 0.012, I 2 = 0.0%, six comparisons, 4,080 patients; Figure 4c).
Urinary tract infections and genital infections
Compared with a placebo, SGLT inhibitors were associated with higher risks of urinary tract infections (RR 1.259, 95% CI 1.034–1.533, P = 0.022, I 2 = 29.3%, 10 comparisons, 7,728 patients; Figure 4d) and genital infections (RR 2.995, 95% CI 1.953–4.594, P < 0.001, I 2 = 61.3%, 10 comparisons, 7,679 participants; Figure 4e). Interestingly, as shown in Figure S5a,b, no significant difference was found in the subgroup analysis of SGLT inhibitors. Therefore, we cannot conclude that either SGLT2 inhibitors or dual SGLT1/2 inhibitors will increase the risk of urinary tract infections (SGLT2 inhibitors: RR 1.105, 95% CI 0.870–1.403, P = 0.414; dual SGLT 1/2 inhibitor: RR 1.417, 95% CI 0.732–2.722, P = 0.296).
Heterogeneity was observed in the analysis of genital infections, and the source of heterogeneity was the study by Satish K Garg (Figure S2b). In the subgroup analysis of genital infections, the results showed that SGLT2 inhibitors were associated with a higher risk of genital infections (RR 3.721, 95% CI 2.741–5.052, P < 0.001) in patients with type 1 diabetes (Figure 4f). As shown in Figure S5c, no significant difference in genital infections was found between the dual SGLT inhibitor and placebo groups (RR 2.297, 95% CI 0.910–5.789, P = 0.078). The present results showed that SGLT inhibitors increased the risk of urinary tract infections in obese (RR 1.590, 95% CI 1.072–2.357, P = 0.021) and younger people (RR 1.421, 95% CI 1.046–1.932, P = 0.025), but no statistically significant difference was found for lean (RR 1.132, 95% CI 0.719–1.782, P = 0.591) and older people (RR 0.977, 95% CI 0.581–1.643, P = 0.930). BMI and age did not influence the effect of SGLT inhibitors on genital infection (Figure S6).
Diarrhea
A total of four studies including 3,118 participants recorded the risk of diarrhea. The meta‐analysis using the fixed effects model showed that compared with the placebo, SGLT inhibitors increased the risk of diarrhea (RR 1.486, 95% CI 1.064–2.075, P = 0.020, I 2 = 40.4%) in patients with type 1 diabetes (Figure S7a). The four studies above all tested the dual SGLT1/2 inhibitor; thus, SGLT2 inhibitors and the dual SGLT1/2 inhibitor could not be compared.
Hypoglycemia
The definition of hypoglycemia was consistent across all RCTs and was in line with current guideline recommendations. For the analysis of the risk of hypoglycemia, no statistical significance was observed, showing no sufficient evidence that the addition of SGLT inhibitors to insulin treatment in patients with type 1 diabetes increases the risk of hypoglycemia (RR 0.980, 95% CI 0.840–1.144, I 2 = 0.0%,P = 0.799, 11 comparisons, 7,756 patients; Figure S7b). In the subgroup analysis, neither type of inhibitor was associated with an increase in the incidence of hypoglycemia (SGLT2 inhibitors: RR 1.150, 95% CI 0.871–1.517, P = 0.325; dual SGLT inhibitor; RR 0.880, 95% CI 0.505–1.534, P = 0.654; Figure S7c,d). The present results showed that the RR value did not change with age and BMI (Figure S8).
Discussion
For the first time, we reported the efficacy and safety of both SGLT2 and dual SGLT1/2 inhibitors for type 1 diabetes, and compared differences between the two types of drugs, age and BMI. We included 13 studies (7,962 participants) published between 2015 and 2019 with a follow‐up duration ranging from 4 to 52 weeks, and five of the studies investigated a dual SGLT 1/2 inhibitor (sotagliflozin). In general, the included literature complied with the principle of random double blindness. Furthermore, no study to date had a larger sample size than the current study. The present analysis showed three notable findings. First, SGLT inhibitors as an add‐on treatment to insulin injections facilitate glycemic control with a decreased insulin dose. Second, SGLT inhibitor treatment is associated with increased incidence rates of urinary tract and genital infections, diarrhea, and DKA, but not hypoglycemia. Third, compared with a placebo, the effects of a dual SGLT inhibitor (sotagliflozin) on type 1 diabetes are consistent with those of SGLT2 inhibitors, but do not increase the risk of genital infections.
Previously, when agents were used as add‐on therapy to insulin in type 1 diabetes treatment, SGLT2 inhibitors were reported to result in significant improvements in glycemia control, decreases in HbA1c, FPG, MAGE and urinary glucose excursion values, and better effects on non‐glucose‐related targets, such as bodyweight loss 37 , 38 , 39 . Some meta‐analyses of SGLT inhibitors have been reported; for example, eight meta‐analyses reported the efficacy and safety of SGLT inhibitors in type 1 diabetes patients (6 for SGLT2 inhibitors, 1 for a dual SGLT1/2 inhibitor and 1 for both) 18 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , and showed that SGLT inhibitors significantly reduced HbA1c levels, FPG levels, insulin doses and MAGE values, which is consistent with the present meta‐analysis.
The two most well‐known members of the SGLT family are SGLT1 and SGLT2. SGLT1, which is mainly expressed in the S3 segment of proximal renal tubules and the brush border of the small intestine, is mainly responsible for 10% of renal sugar reabsorption, and intestinal glucose and galactose absorption. SGLT2 is specifically expressed in the S1 segment of proximal renal tubules and is responsible for 90% of renal sugar reabsorption 45 . Thus, when SGLT1 is inhibited, glucose in the intestine is blocked, and glucose is broken down into short‐chain fatty acids by bacteria at the distal end of the small intestine, promoting the secretion and release of glucagon‐like peptide‐1 and peptide YY by L cells at the distal end of the intestine 46 . In contrast, attenuating SGLT2 decreases renal glucose reabsorption. Compared with SGLT2 inhibitors, sotagliflozin, a dual inhibitor, blocks SGLT1 and SGLT2 simultaneously, reducing the reabsorption of glucose by the kidneys and the intestine, which is beneficial for decreasing postprandial blood glucose. The present meta‐analysis showed the hypoglycemic effect of a dual SGLT1/2 inhibitor is consistent with that of SGLT2 inhibitors, and does not increase the occurrence of adverse events. Therefore, oral hypoglycemic agents, such as SGLT inhibitors, might be used in type 1 diabetes patients. At present, just 30% of patients with type 1 diabetes have achieved the goal of blood glucose control, and 20 and 40% have hypoglycemia and are overweight, respectively. In addition to insulin, there are few effective drugs for type 1 diabetes. Therefore, the demand for additional drug treatment for type 1 diabetes is far from being met. For type 1 diabetes patients with overweight/obesity receiving the best insulin treatment, but still not meeting blood sugar targets, SGLT inhibitors might indeed become a good supplement for insulin.
Regardless, the adverse reactions caused by SGLT inhibitors should still be considered. SGLT inhibitors can promote the excretion of a large amount of glucose through the urine and increase glucose concentration in the genitourinary tract, thus increasing the risk of bacterial and fungal infection in patients. Furthermore, the mechanism of increasing the risk of DKA is likely as follows. First, the agents might influence the secretion of insulin and glucagon, and promote the decomposition of adipose tissue and beta‐oxidation of fatty acids, thus increasing the formation of ketones in the liver 47 . Second, SGLT inhibitors can increase lipid mobilization and free fatty acid oxidation in vivo, increase the level of free fatty acid and 13‐hydroxybutyric acid in plasma, reduce the elimination of ketone bodies by the kidneys, and increase the reabsorption of ketone bodies in the proximal convoluted tubules 48 . Thus, SGLT inhibitors should be used with caution in type 1 diabetes patients with recurrent urogenital infections, ketosis or acidosis.
There were still several limitations of our analysis. First, the present number of studies included was not sufficient for a dose subgroup analysis. Second, there was still heterogeneity in some analyses. Third, we searched only the English and Chinese literature in this research, and did not consider all studies from all countries worldwide. Fourth, the review process also did not include gray literature.
The present meta‐analysis analyzed SGLT2 inhibitors and dual SGLT1/2 inhibitors as supplementary therapy for patients with type 1 diabetes, and proper subgroup analyses between SGLT2 inhibitors and dual SGLT1/2 inhibitors were carried out, showing the potential benefits of SGLT inhibitors, such as better glycemic control. Furthermore, the present meta‐analysis, which provides strong evidence for oral medication for patients with type 1 diabetes, reminds clinicians that such medications should be used with caution in patients with ketosis. Finally, our results are conducive to further exploration of oral drugs in patients with type 1 diabetes.
In conclusion, SGLT inhibitors are associated with desirable glycemic control, and the effects of SGLT2 inhibitors and dual SGLT1/2 inhibitors were found to be consistent in the present meta‐analysis, except for the risk of genital infections. Aiming to provide more solid evidence regarding the use of SGLT inhibitors for the treatment of type 1 diabetes, larger‐scale clinical trials are required to further investigate the therapeutic value of SGLT2 inhibitors, dual SGLT1/2 inhibitors and even SGLT1 inhibitors in patients with type 1 diabetes.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | Forest illustration of body mass index and age subgroups meta‐analysis on fasting plasma glucose and glycated hemoglobin.
Figure S2 | (a) Heterogeneity of daily total insulin dose. (b) Heterogeneity of genital infection.
Figure S3 | Forest illustration of body mass index and age subgroups meta‐analysis of insulin dose.
Figure S4 | Forest illustration of body mass index and age subgroups meta‐analysis of diabetic ketoacidosis.
Figure S5 | Forest illustration of drug subgroup meta‐analysis of genitourinary tract infections.
Figure S6 | Forest illustration of body mass index and age subgroups meta‐analysis of genitourinary tract infections.
Figure S7 | Forest illustration of meta‐analysis on diarrhea and hypoglycemia.
Figure S8 | Forest illustration of body mass index and age subgroups meta‐analysis of hypoglycemia.
Table S1 | Quality assessment of included studies.
Acknowledgments
All authors contributed significantly and met the criteria of authorship. This work was supported by the National Science Foundation of Hunan Province for Excellent Young Scholars (2020JJ3056 to Y Xiao), the National Natural Science Foundation of China (NSFC) grant (81870577, 81670772 to Y Xiao, 81800745 to C Deng), National Key Research and Development Project (2016YFC1305000, 2016YFC1305001 to ZG Zhou), and Science and Technology Major Project of Hunan Province (2017SK1020 to ZG Zhou).
J Diabetes Investig. 2021
References
- 1. Dimeglio LA, Evans‐molina C. Type 1 diabetes. Lancet 2019; 391: 2449–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiang JL, Kirkman MS, Laffel LMB, et al. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014; 37: 2034–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aschner P, Horton E, Leiter LA, et al. Practical steps to improving the management of type 1 diabetes: recommendations from the Global Partnership for Effective Diabetes Management. Int J Clin Pract 2010; 64: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physician 2005; 71: 1705–1714. [PubMed] [Google Scholar]
- 5. Nathan DM, Cleary PA, Backlund J‐YC, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Association AD. Microvascular complications and foot care: standards of medical care in diabetes – 2018. Diabetes Care 2018; 41: 105–118. [DOI] [PubMed] [Google Scholar]
- 7. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care 2015; 38: 971–978. [DOI] [PubMed] [Google Scholar]
- 8. Association AD. Glycemic targets: standards of medical care in diabetes – 2018. Diabetes Care 2018; 41: 55–64. [DOI] [PubMed] [Google Scholar]
- 9. Nathan D, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Munir KM, Davis SN. The treatment of type 1 diabetes mellitus with agents approved for type 2 diabetes mellitus. Expert Opin Pharmacother 2015; 16: 2331–2341. [DOI] [PubMed] [Google Scholar]
- 11. Lüddeke HJ, Sreenan S, Aczel S, et al. PREDICTIVETM ‐ A global, prospective observational study to evaluate insulin detemir treatment in types 1 and 2 diabetes: baseline characteristics and predictors of hypoglycaemia from the European cohort. Diabetes Obes Metab 2007; 9: 428–434. [DOI] [PubMed] [Google Scholar]
- 12. Donnelly LA, Morris AD, Frier BM, et al. Frequency and predictors of hypoglycaemia in Type 1 and insulin‐treated Type 2 diabetes: a population‐based study. Diabet Med 2005; 22: 749–755. [DOI] [PubMed] [Google Scholar]
- 13. Comee M, Peters A. The changing therapeutic armamentarium for patients with type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 2016; 23: 106–110. [DOI] [PubMed] [Google Scholar]
- 14. Smith LB, Liu X, Johnson SB, et al. Family adjustment to diabetes diagnosis in children: can participation in a study on type 1 diabetes genetic risk be helpful? Pediatr Diabetes 2018; 19: 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elding Larsson H, Lynch KF, Lönnrot M, et al. Pandemrix® vaccination is not associated with increased risk of islet autoimmunity or type 1 diabetes in the TEDDY study children. Diabetologia 2018; 61: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Control TD, Trial C. Hypoglycemia in the diabetes control and complications trial. Diabetes 1997; 46: 271–286. [PubMed] [Google Scholar]
- 17. Ayadi H. Towards a reconstruction of african american identity in Toni Morrison’s beloved Hajer Ayadi University of Arts and Humanities of Sfax. JAMA 2011; 1: 263–270. [Google Scholar]
- 18. Yamada T, Shojima N, Noma H, et al. Sodium‐glucose co‐transporter‐2 inhibitors as add‐on therapy to insulin for type 1 diabetes mellitus: systematic review and meta‐analysis of randomized controlled trials. Diabetes Obes Metab 2018; 20: 1755–1761. [DOI] [PubMed] [Google Scholar]
- 19. Wu JHY, Foote C, Blomster J, et al. Effects of sodium‐glucose cotransporter‐2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2016; 4: 411–419. [DOI] [PubMed] [Google Scholar]
- 20. Ji L, Li H, Guo X, et al. Impact of baseline BMI on glycemic control and weight change with metformin monotherapy in Chinese type 2 diabetes patients: phase IV open‐label. Trial 2013; 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahmad OB, Boschi‐pinto C, Lopez AD. Age standardization of rates : a new who standard GPE Discussion Paper Series: No. 31 EIP/GPE/EBD World Health Organization 2001. 2001.
- 22. Grant J, Hunter A. Measuring inconsistency in knowledgebases. J Intell Inf Syst 2006; 27: 159–184. [Google Scholar]
- 23. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT‐1): 24 week results from a multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 864–876. [DOI] [PubMed] [Google Scholar]
- 24. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT‐1 52‐week study. Diabetes Care 2018; 41: 2552–2559. [DOI] [PubMed] [Google Scholar]
- 25. Kuhadiya ND, Ghanim H, Mehta A, et al. Dapagliflozin as additional treatment to liraglutide and insulin in patients with type 1 diabetes. J Clin Endocrinol Metab 2016; 101: 3506–3515. [DOI] [PubMed] [Google Scholar]
- 26. Mathieu C, Dandona P, Gillard P, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT‐2 Study): 24‐week results from a randomized controlled trial. Diabetes Care 2018; 41: 1938–1946. [DOI] [PubMed] [Google Scholar]
- 27. Henry RR, Thakkar P, Tong C, et al. Efficacy and safety of canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, as add‐on to insulin in patients with type 1 diabetes. Diabetes Care 2015; 38: 2258–2265. [DOI] [PubMed] [Google Scholar]
- 28. Pieber TR, Famulla S, Eilbracht J, et al. Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4‐week, randomized, placebo‐controlled trial (EASE‐1). Diabetes Obes Metab 2015; 17: 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenstock J, Marquard J, Laffel LM, et al. Empagliflozin as adjunctive to insulin therapyin type 1 diabetes: the EASE trials. Diabetes Care 2018; 41: 2560–2569. [DOI] [PubMed] [Google Scholar]
- 30. Kaku K, Isaka H, Sakatani T, et al. Efficacy and safety of ipragliflozin add‐on therapy to insulin in Japanese patients with type 1 diabetes mellitus: a randomized double‐blind, phase 3 trial. Diabetes Obes Metab 2019; 21: 2284–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sands AT, Zambrowicz BP, Rosenstock J, et al. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care 2015; 38: 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garg SK, Henry RR, Banks P, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 2017; 377: 2337–2348. [DOI] [PubMed] [Google Scholar]
- 33. Danne T, Cariou B, Banks P, et al. HbA1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European in Tandem2 study. Diabetes Care 2018; 41: 1981–1990. [DOI] [PubMed] [Google Scholar]
- 34. Buse JB, Garg SK, Rosenstock J, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American in Tandem1 study. Diabetes Care 2018; 41: 1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baker C, Wason S, Banks P, et al. Dose‐dependent glycometabolic effects of sotagliflozin on type 1 diabetes over 12 weeks: the in Tandem4 trial. Diabetes Obes Metab 2019; 21: 2440–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Owens DK, Lohr KN, Atkins D, et al. AHRQ Series Paper 5: grading the strength of a body of evidence when comparing medical interventions‐Agency for Healthcare Research and Quality and the Effective Health‐Care Program. J Clin Epidemiol 2010; 63: 513–523. [DOI] [PubMed] [Google Scholar]
- 37. El Masri D, Ghosh S, Jaber LA. Safety and efficacy of sodium‐glucose cotransporter 2 (SGLT2) inhibitors in type 1 diabetes: a systematic review and meta‐analysis. Diabetes Res Clin Pract 2018; 137: 83–92. [DOI] [PubMed] [Google Scholar]
- 38. Chen J, Fan F, Wang JY, et al. The efficacy and safety of SGLT2 inhibitors for adjunctive treatment of type 1 diabetes: a systematic review and meta‐analysis. Sci Rep 2017; 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bonifacio E, Beyerlein A, Hippich M, et al. Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: a prospective study in children. PLoS Medicine 2018; 15: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Y, Chen S, Pan H, et al. Safety and efficiency of SGLT2 inhibitor combining with insulin in subjects with diabetes. Medicine 2017; 96: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang W, Leil TA, Johnsson E, et al. Comparison of the pharmacokinetics and pharmacodynamics of dapagliflozin in patients with type 1 versus type 2 diabetes mellitus. Diabetes Obes Metab 2016; 18: 236–240. [DOI] [PubMed] [Google Scholar]
- 42. Yang Y, Pan H, Wang B, et al. Efficacy and safety of SGLT2 inhibitors in patients with type 1 diabetes: a meta‐analysis of randomized controlled trials. Chinese Med Sci J 2017; 32: 22–27. [DOI] [PubMed] [Google Scholar]
- 43. Lu J, Tang L, Meng H, et al. Effects of sodium‐glucose cotransporter (SGLT) inhibitors in addition to insulin therapy on glucose control and safety outcomes in adults with type 1 diabetes: a meta‐analysis of randomized controlled trials. Diabetes Metab Res Rev 2019; 35: 1–10. [DOI] [PubMed] [Google Scholar]
- 44. Musso G, Gambino R, Cassader M, et al. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta‐analysis of randomised controlled trials. BMJ 2019; 365: l1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee YJ, Lee YJ, Han HJ. Regulatory mechanisms of Na+/glucose cotransporters in renal proximal tubule cells. Kidney Int 2007; 72(SUPPL. 106): 27–35. [DOI] [PubMed] [Google Scholar]
- 46. Zhou J, Martin RJ, Tulley RT, et al. Dietary resistant starch upregulates total GLP‐1 and PYY in a sustained day‐long manner through fermentation in rodents. Am J Physiol Endocrinol Metab 2008; 295: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Palmer BF, Clegg DJ, Taylor SI, et al. Diabetic ketoacidosis, sodium glucose transporter‐2 inhibitors and the kidney. J Diabetes Complications 2016; 30: 1162–1166. [DOI] [PubMed] [Google Scholar]
- 48. Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev 2017; 33: 1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Forest illustration of body mass index and age subgroups meta‐analysis on fasting plasma glucose and glycated hemoglobin.
Figure S2 | (a) Heterogeneity of daily total insulin dose. (b) Heterogeneity of genital infection.
Figure S3 | Forest illustration of body mass index and age subgroups meta‐analysis of insulin dose.
Figure S4 | Forest illustration of body mass index and age subgroups meta‐analysis of diabetic ketoacidosis.
Figure S5 | Forest illustration of drug subgroup meta‐analysis of genitourinary tract infections.
Figure S6 | Forest illustration of body mass index and age subgroups meta‐analysis of genitourinary tract infections.
Figure S7 | Forest illustration of meta‐analysis on diarrhea and hypoglycemia.
Figure S8 | Forest illustration of body mass index and age subgroups meta‐analysis of hypoglycemia.
Table S1 | Quality assessment of included studies.


