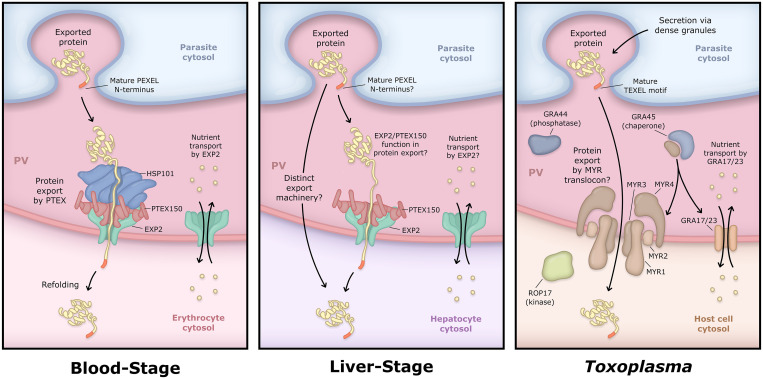
Fig 5. Comparison of PV transport in Plasmodium blood and liver-stages and Toxoplasma.
In contrast to the blood-stage, HSP101 has not been detected in the liver-stage PV, suggesting fundamental differences in the export mechanism within the hepatocyte. While EXP2 has recently been implicated in sporozoite invasion of hepatocytes, it is unknown whether EXP2 and/or PTEX150 contribute to protein export or small molecule transport during intrahepatic development. While EXP2 orthologs GRA17 and 23 are required for small molecule transport across the Toxoplasma PVM, they are not involved in the export of dense granule effector proteins from the PV. Instead, a distinct set of proteins are required for export of these effectors including the membrane proteins MYR1–4 which are believed to form a novel translocon (possible MYR translocon organization shown here is purely speculative for purposes of illustration). Export also requires the putative phosphatase GRA44 and the chaperone GRA45, which may insert the MYR proteins into the PVM. Export additionally depends on the activity of ROP17, a kinase injected from the rhoptries into the host cytosol during invasion. ROP17 acts at the PV surface but the phosphorylated target(s) is not known. TgPPMC3, an additional phosphatase localized to the PV and involved in the export of a subset of effectors, is not represented in the cartoon. Processing of a TEXEL motif by the aspartic protease ASP5 is important for export of some Toxoplasma dense granule effectors while others lack the motif and are not processed by ASP5, although their export still requires ASP5 for unclear reasons. In contrast to the Plasmodium blood-stage, most ASP5 substrates are non-exported PV resident proteins, including MYR1, GRA44, and GRA45. EXP2, exported protein 2; HSP101, heat shock protein 101; PTEX, Plasmodium Translocon of EXported proteins; PV, parasitophorous vacuole; PVM, PV membrane; TEXEL, Toxoplasma EXport ELement.