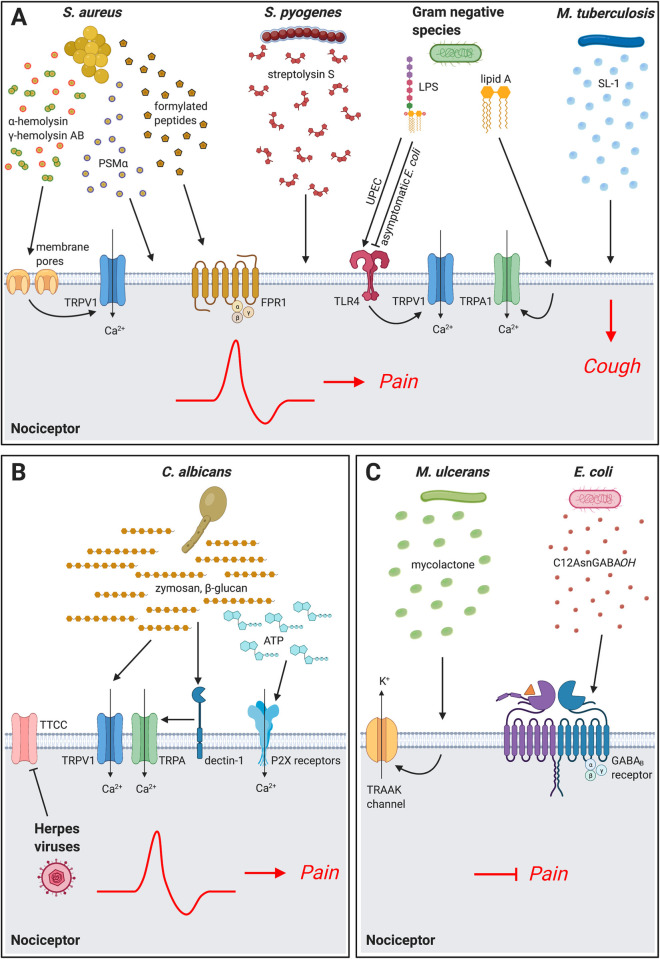
Fig 1. Microbial mechanisms that act on nociceptor neurons to mediate pain and cough.
(A) Bacterial pathogens directly activate nociceptors to cause pain and cough during infection. S. aureus produces the pore-forming toxins α-hemolysin, bicomponent leukocidin HlgAB, and PSMα, which act on sensory neurons to induce cation influx, action potential generation, and pain production. S. aureus formylated peptides bind to FPR1 receptors on nociceptors to induce mechanical pain sensitivity. S. pyogenes produces the cytolytic toxin SLS to activate nociceptors and produce pain during infection. LPS from gram-negative bacteria including UPEC can act through TLR4 expressed on nociceptors to sensitize the TRPV1 ion channel. LPS from several gram-negative bacterial species also directly activates the nociceptive ion channel TRPA1, an activity that depends on the lipid A moiety. By contrast, LPS from asymptomatic E. coli can block pain. M. tuberculosis produces SL-1, which induces calcium influx in lung-innervating nociceptors and mediates cough during infection. (B) Fungal and viral mechanisms of pain. C. albicans cell wall components zymosan and β-glucan can activate neurons to produce pain. β-glucan can directly activate neurons via the dectin-1 receptor expressed by nociceptors. β-glucan also stimulates keratinocytes to release ATP, which subsequently activates P2X receptors on neurons to produce pain. Herpesviruses infect nociceptor neurons, reducing the expression of TTCC, which alters electrical excitability of neurons. (C) Microbial products that silence neurons and pain. M. ulcerans alters nociceptor signaling through a mycolactone that induces potassium efflux via TRAAK family potassium channels. E. coli Nissle 1917 secretes the C12AsnGABAOH lipopeptide which inhibits nociceptor activation and pain via the GABAB receptor. Created with BioRender.com. FPR, formyl peptide receptor; LPS, lipopolysaccharide; PSMα, phenol soluble modulin α3; SL-1, sulfolipid-1; SLS, streptolysin S; TLR, Toll-like receptor; TRP, transient receptor potential; TTCC, T-type calcium channels; UPEC, uropathogenic Escherichia coli.