Abstract
Objectives
This study aimed to investigate the psychological distress and its associated factors among cancer survivors in Malaysia during the COVID-19 pandemic.
Methods
An anonymous Internet-based study was conducted between 23 April and 26 June 2020. During the study period, the country underwent phase 3 and phase 4 of the Movement Control Order (MCO), Conditional Movement Control Order (CMCO), and Recovery Movement Control Order (RMCO). Psychological distress was measured using the Hospital Anxiety and Depression Scale (HADS), which is a 14-item self-assessment scale for measuring distress (total HADS score; HADS-T) with two subscales, namely, anxiety (HADS-A) and depression (HADS-D). Perceived threat of infection was measured based on the health belief model.
Results
From a total of 631 responses received, the proportion of participants with anxiety and depression symptoms (above threshold score of 8 on HADS-A and HADS-D) was 29.0 and 20.9%, respectively. Psychological distress (HADS-T > 16) was reported in 22.3% of the respondents. A total of 16.5% had combined anxiety and depression symptoms. The highest HADS-A (6.10; 95% CI 5.64–6.56), HADS-D (5.61; 95% CI 5.14–6.08), and HADS-T (11.71; 95% CI 10.84–12.58) scores were reported among respondents during phase 4 of the MCO. Partial least square-based structural equation modelling (PLS-SEM) revealed that self-perceived health status, perceived susceptibility, and severity of COVID-19 have the greatest effect, leading to higher HADS-A, HADS-D, and HADS-T scores.
Conclusion
Heightened psychological distress was evident in cancer survivors particularly during the enforcement of the MCO over COVID-19. Providing support to address cancer survivors’ psychological and emotional needs during the COVID-19 pandemic is essential.
Keywords: Cancer survivors, COVID-19 pandemic, Psychological distress
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) in Wuhan, China, in the late December of 2019, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1, 2], has spread rapidly within China, to other neighbouring countries in Asia and beyond. In Malaysia, the SARS-CoV-2 infection was first reported on 25 January 2020. Nonetheless, the number of cases was relatively low for over a month, and cases were mainly confined to imported ones. Localized clusters began to be found in early March, and by 17 March, the number of confirmed cases in Malaysia had reached 673; this is also the date when the first two fatalities were reported in the country. Subsequently, a nationwide Movement Control Order (MCO) to prevent further spread of the coronavirus was implemented on 18 March. By the end of March, Malaysia had the largest cumulative confirmed number of coronavirus cases in South East Asia [3]. In Malaysia, the MCO order included the closure of schools and higher education institutions and ‘non-essential’ businesses, as well as a general prohibition of mass movements and large gathering. The country has gone through the four MCO phases, with all the strict actions recommended by the World Health Organization (WHO) to contain the outbreak. A Conditional Movement Control Order (CMCO) was implemented from 13 May to 9 June, and a Recovery Movement Control Order (RMCO) took effect from 10 June and last until 31 August with more lenient restrictions. Most recently the RMCO has been extended until the end of 2020.
Movement restrictions and social distancing measures to flatten the curve of COVID-19 spread have exacerbated the emotional vulnerability of cancer patients and survivors [4]. The COVID-19 pandemic has also tremendously impacted the spectrum of cancer care management [4, 5]. Cancer diagnosis and treatment represent significant stressors and a traumatizing event for many patients. For most patients, whose immune systems may already be compromised, psychological distress associated with the pandemic may further weaken their immune system [6]. Secondly, people who are immunocompromised, whether caused by the disease itself or the treatment, have an increased risk of SARS-CoV-2 infection compared with the general population [7, 8]. Further, there is also evidence showing that cancer history confers the highest risk of severe complications and is correlated with poorer outcomes from SARS-CoV-2 infections [9]. Recent evidence indicates that depressive symptoms persist over time in cancer patients and survivors, suggesting the psychological vulnerability of cancer survivors’ years post-diagnosis [10]. Therefore, cancer patients and cancer survivors pose unique management dilemmas during infectious disease pandemics like COVID-19.
According to the Malaysia National Cancer Registry report (MNCRR) 2012–2016, cancer cases in Malaysia have been increasing for the past 10 years, and cancer remains the second highest cause of death [11]. Cancer cases in Malaysia increased to 115,238 from 2012 to 2016, compared to 103,507 from 2007 to 2011 [11]. Due to the growing number of cancer cases, and in addition to advances in early detection and improved cancer treatment that increased the life expectancy of cancer patients, the number of cancer survivors in Malaysia is large. Hence, the psychological burden among cancer survivors in Malaysia during the COVID-19 pandemic warrants investigation. It is important to regularly assess their psychological status and facilitate timely intervention when necessary.
The primary objectives of the present study, therefore, were to examine the psychological distress of people who have survived cancer during the COVID-19 outbreak in Malaysia. Factors such as socio-demographics, the characteristics of cancer [12, 13], and the risk perception of COVID-19 [14] can affect psychological distress during the pandemic. Thus, understanding the extent to which these factors influence psychological distress will also be investigated.
Methods
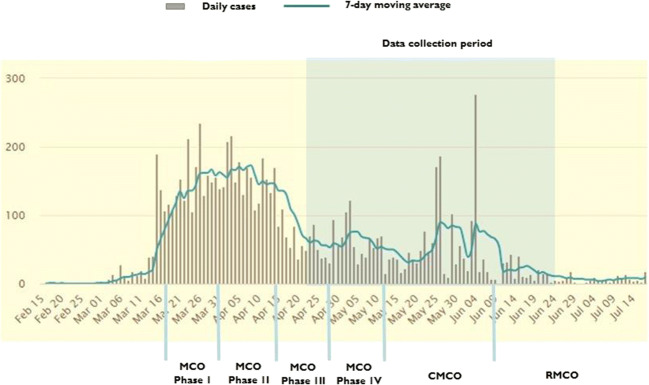
An anonymous Internet-based study was conducted between 23 April and 26 June 2020. The data collection period, the trend of the confirmed COVID-19 cases in Malaysia, and MOC phases are shown in Fig. 1. Inclusion criteria for this study were as follows: (1) being older than 18 years of age; (2) having at least 2 years of diagnosis; (3) currently not receiving treatment except for endocrine therapy, and (4) being a Malaysian citizen. The researchers used social network platforms (Facebook, Instagram, and WhatsApp) to disseminate and advertise the survey link to the general public. The survey link was also sent to leaders of the local community-based cancer support groups to disseminate to their members. Members of the cancer support groups who received the survey link were encouraged to share the link with their friends or acquaintances. All respondents were informed that their participation was voluntary. Informed consent was obtained using an online consent form that each participant had to actively agree to sign.
Fig. 1.
Data collection period of the survey, confirmed cases of COVID-19 in Malaysia, and stages of Movement Control Order in Malaysia
Ethical considerations
This study was approved by the University of Malaya Research Ethics Committee (UMREC), approval code UM.TNC2/UMREC – 885.
Instruments
The survey consisted of sections of questions that assessed (1) socio-demographic background, characteristics of cancer, and perceived health status; (2) perceived threat of the coronavirus infection; and (3) psychological distress measured using the Hospital Anxiety and Depression Scale (HADS).
Personal details, including age, gender, ethnicity, educational level, and average monthly household income, were queried. Baseline information about cancer characteristics, namely, time since cancer diagnosis, type of cancer, stage of cancer at the time of first diagnosis, type of cancer treatment received, and comorbidities, were queried. Overall perceived health is a subjective, individualized self-assessment of the current overall state of personal health and was measured by a single question asking for a rating of current general health status using four item choices (‘excellent’, ‘good’, ‘fair’, or ‘poor’).
We used the health belief model, which explores two dimensions: perceived susceptibility (three-item questions) and perceived severity (three-item questions). Perceived susceptibility addressed one’s subjective perception of the risk of contracting SARS-CoV-2. Perceived severity assessed feelings concerning the seriousness of SARS-CoV-2 infection. The combination of perceived susceptibility and severity is referred to as ‘perceived threat’ and is one of the core constructs in the health belief model [15–17]. The response options were ‘strongly agree’, ‘agree’, ‘disagree’, or ‘strongly disagree’. The questions of perceived threat are shown in the first column of Table 1.
Table 1.
Participant characteristics by mean HADS-A, HADS-D, and HADS-T scores (n = 631)
| N (%) | Total score (Mean ± SD) |
|||
|---|---|---|---|---|
| Demographics | HADS-A | HADS-D | HADS-T | |
| Age group | ||||
| 21–49 | 160 (25.4) | 5.72 ± 4.05 | 3.84 ± 3.38 | 9.56 ± 7.02 |
| 50–60 | 230 (36.5) | 5.03 ± 3.60 | 3.96 ± 3.50 | 9.00 ± 6.49 |
| 61–86 | 241 (38.2) | 5.90 ± 3.95 | 5.25 ± 3.95 | 11.15 ± 7.33 |
| 0.042 | p < 0.001 | 0.003 | ||
| Gender | ||||
| Male | 174 (27.6) | 5.72 ± 3.92 | 5.51 ± 3.86 | 11.29 ± 7.25 |
| Female | 457 (72.4) | 5.47 ± 3.85 | 4.01 ± 3.56 | 9.48 ± 6.86 |
| 0.454 | p < 0.001 | 0.005 | ||
| Ethnicity | ||||
| Malay | 174 (27.6) | 5.43 ± 3.89 | 4.18 ± 3.75 | 9.62 ± 7.14 |
| Chinese | 332 (52.6) | 5.09 ± 3.76 | 3.80 ± 3.22 | 8.89 ± 6.42 |
| Indian | 31 (4.9) | 6.58 ± 4.24 | 4.61 ± 3.36 | 11.19 ± 7.08 |
| Indigenous of Sabah/Sarawak | 94 (14.9) | 6.97 ± 3.73 | 7.01 ± 4.25 | 13.98 ± 7.36 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
| Highest educational level | ||||
| Secondary school and below | 225 (35.7) | 6.28 ± 3.83 | 5.57 ± 4.26 | 11.86 ± 7.54 |
| College/university | 406 (64.3) | 5.12 ± 3.83 | 3.79 ± 3.19 | 8.91 ± 6.47 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
| Average monthly household income (MYR) | ||||
| 4000 and below | 204 (32.3) | 5.36 ± 3.84 | 4.69 ± 3.96 | 10.05 ± 7.22 |
| 4001–8000 | 275 (43.6) | 6.17 ± 3.92 | 4.85 ± 3.76 | 11.02 ± 7.21 |
| > 8000 | 152 (24.1) | 4.63 ± 3.60 | 3.29 ± 2.95 | 7.92 ± 5.87 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
| Cancer characteristics | ||||
| Time since diagnosis (years) | ||||
| 4 and below | 254 (40.3) | 5.46 ± 3.66 | 3.89 ± 3.29 | 9.35 ± 6.36 |
| 5–9 | 256 (40.6) | 5.76 ± 4.31 | 4.80 ± 3.97 | 10.56 ± 7.80 |
| 10 years and above | 121 (19.1) | 5.22 ± 3.23 | 4.75 ± 3.83 | 9.98 ± 6.48 |
| 0.415 | 0.012 | 0.151 | ||
| Type of cancer | ||||
| Breast | 390 (61.8) | 5.13 ± 3.76 | 3.64 ± 3.23 | 8.76 ± 6.42 |
| Prostate | 164 (26.0) | 5.73 ± 3.85 | 5.63 ± 3.84 | 11.37 ± 7.16 |
| Bladder | 22 (3.5) | 11.14 ± 2.49 | 11.41 ± 2.81 | 22.55 ± 4.87 |
| Ovarian | 13 (2.1) | 6.69 ± 3.95 | 5.85 ± 3.41 | 12.54 ± 6.40 |
| Cervical | 12 (1.9) | 5.00 ± 3.02 | 3.58 ± 2.78 | 8.58 ± 5.28 |
| Nasopharyngeal | 8 (1.3) | 4.63 ± 3.89 | 3.00 ± 1.69 | 7.63 ± 5.45 |
| Lymphoma | 7 (1.1) | 6.29 ± 3.50 | 2.43 ± 2.57 | 8.71 ± 5.85 |
| Colon | 5 (0.8) | 6.00 ± 1.41 | 3.40 ± 0.89 | 9.40 ± 2.19 |
| Appendix | 4 (0.6) | 5.50 ± 3.00 | 1.75 ± 1.50 | 7.25 ± 1.50 |
| Others | 6 (1.0) | 5.00 ± 4.20 | 2.33 ± 2.58 | 7.33 ± 6.59 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
| Stage of cancer at the time of first diagnosis | ||||
| 0 | 41 (6.5) | 3.76 ± 2.76 | 2.66 ± 2.73 | 6.42 ± 4.88 |
| 1 | 190 (30.1) | 4.92 ± 3.65 | 4.04 ± 3.22 | 8.96 ± 6.38 |
| 2 | 246 (39.0) | 5.82 ± 3.71 | 4.83 ± 4.08 | 10.65 ± 7.23 |
| 3 | 123 (19.5) | 6.17 ± 4.10 | 4.72 ± 3.82 | 10.89 ± 7.43 |
| 4 | 31 (4.9) | 6.94 ± 5.29 | 4.71 ± 3.25 | 11.65 ± 7.67 |
| p < 0.001 | 0.004 | p < 0.001 | ||
| Type of treatment received | ||||
| Surgery | ||||
| Yes | 571 (90.5) | 5.56 ± 3.83 | 4.49 ± 3.76 | 10.05 ± 7.02 |
| No | 60 (9.5) | 5.32 ± 4.18 | 3.78 ± 3.05 | 9.10 ± 6.92 |
| 0.643 | 0.160 | 0.318 | ||
| Chemotherapy | ||||
| Yes | 404 (64.0) | 6.12 ± 3.92 | 4.96 ± 3.96 | 11.08 ± 7.26 |
| No | 227 (36.0) | 4.51 ± 3.55 | 3.47 ± 2.97 | 7.97 ± 6.06 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
| Radiotherapy | ||||
| Yes | 394 (62.4) | 5.96 ± 3.89 | 4.91 ± 3.97 | 10.87 ± 7.31 |
| No | 237 (37.6) | 4.84 ± 3.73 | 3.62 ± 3.05 | 8.46 ± 6.20 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
| Endocrine therapy | ||||
| Yes | 209 (33.1) | 5.62 ± 3.71 | 5.29 ± 3.71 | 10.91 ± 6.84 |
| No | 422 (66.9) | 5.50 ± 3.95 | 3.99 ± 3.63 | 9.49 ± 7.05 |
| 0.715 | p < 0.001 | 0.017 | ||
| Targeted therapy | ||||
| Yes | 77 (12.2) | 6.00 ± 4.60 | 4.55 ± 3.82 | 10.55 ± 7.75 |
| No | 554 (87.8) | 5.47 ± 3.75 | 4.41 ± 3.69 | 9.88 ± 6.91 |
| 0.263 | 0.757 | 0.435 | ||
| Alternative therapy | ||||
| Yes | 44 (7.0) | 5.96 ± 3.85 | 4.09 ± 3.03 | 10.05 ± 6.44 |
| No | 587 (93.0) | 5.51 ± 3.87 | 4.45 ± 3.75 | 9.95 ± 7.06 |
| 0.458 | 0.538 | 0.934 | ||
| Comorbidities | ||||
| Diagnosed with other comorbidities | ||||
| Yes | 200 (31.7) | 6.39 ± 4.09 | 5.76 ± 4.23 | 12.15 ± 7.71 |
| No | 431 (68.3) | 5.14 ± 3.70 | 3.81 ± 3.26 | 8.95 ± 6.42 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
| Overall health status | ||||
| Self-perceived overall health status | ||||
| Excellent | 84 (13.3) | 2.73 ± 2.92 | 1.81 ± 2.44 | 4.54 ± 4.89 |
| Good | 314 (49.8) | 4.83 ± 3.42 | 3.37 ± 2.93 | 8.19 ± 5.74 |
| Fair | 202 (32.0) | 7.04 ± 3.55 | 5.97 ± 3.10 | 13.01 ± 6.03 |
| Poor | 31 (4.9) | 10.58 ± 3.84 | 12.13 ± 3.12 | 22.71 ± 5.77 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
| Health belief model | ||||
| Perceived susceptibility | ||||
| My chance of getting coronavirus disease in the next few months is great | ||||
| Strongly agree/agree | 337 (53.4) | 6.21 ± 3.98 | 5.15 ± 4.04 | 11.37 ± 7.52 |
| Strongly disagree/disagree | 294 (46.6) | 4.76 ± 3.59 | 3.59 ± 3.08 | 8.35 ± 6.00 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
| I am worried about the likelihood of getting coronavirus disease | ||||
| Strongly agree/agree | 442 (70.0) | 6.24 ± 3.89 | 5.01 ± 3.87 | 11.25 ± 7.23 |
| Strongly disagree/disagree | 189 (30.0) | 3.89 ± 3.26 | 3.05 ± 2.86 | 6.95 ± 5.40 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
| Getting coronavirus disease is currently a possibility for me | ||||
| Strongly agree/agree | 364 (57.7) | 6.37 ± 3.97 | 5.32 ± 3.94 | 11.69 ± 7.41 |
| Strongly disagree/disagree | 267 (42.3) | 4.40 ± 3.41 | 3.20 ± 29.4 | 7.60 ± 5.63 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
| Perceived severity | ||||
| Complications from coronavirus disease are serious | ||||
| Strongly agree/agree | 604 (95.7) | 5.66 ± 3.85 | 4.53 ± 3.72 | 10.19 ± 7.00 |
| Strongly disagree/disagree | 27 (4.3) | 2.82 ± 3.13 | 2.08 ± 2.42 | 4.89 ± 5.29 |
| p < 0.001 | 0.001 | p < 0.001 | ||
| I will be very sick if I get coronavirus disease | ||||
| Strongly agree/agree | 523 (82.9) | 5.91 ± 3.89 | 4.84 ± 3.75 | 10.74 ± 7.06 |
| Strongly disagree/disagree | 108 (17.1) | 3.74 ± 3.22 | 2.44 ± 2.68 | 6.18 ± 5.39 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
| I am afraid of getting coronavirus disease | ||||
| Strongly agree/agree | 565 (89.5) | 5.81 ± 3.85 | 4.64 ± 3.77 | 10.45 ± 7.04 |
| Strongly disagree/disagree | 66 (10.5) | 3.23 ± 3.16 | 2.58 ± 2.40 | 5.80 ± 5.13 |
| p < 0.001 | p < 0.001 | p < 0.001 | ||
Psychological distress was measured using the HADS [18]. The HADS is a valid and reliable self-rating scale that measures anxiety and depression in both hospitals and communities. HADS is the most extensively validated scale for screening emotional distress in cancer patients [19]. It is a 14-item scale with seven items each for anxiety and depression subscales. Each of the subscales, namely, HADS-A (anxiety) and HADS-D (depression), consists of seven items, and both of them had scores ranging from 0 (no problems) to 3 (maximum distress) resulting in a sum score ranging from 0 to 21 for both anxiety and depression. Five of the 14 items were reverse coded. For HADS-A and HADS-D, a subscale score > 8 denotes anxiety or depression, and a HADS total score (HADS-T) > 16 denotes psychological distress [18]. The questionnaire is available in two different languages, English and Bahasa Malaysia, the native language in Malaysia. The original HADS questionnaire in English was translated into Bahasa Malaysia. A standard forward and back-translation procedure was followed.
Statistical analyses
Descriptive statistics were performed for continuous variables, and frequency distribution and 95% confidence interval were used to define the distribution of categorical variables. An independent sample t-test was used to find the difference in means of HADS scores between two independent groups. One-way analysis of variance (ANOVA) was used to determine whether there are any significant differences between the means of HADS scores of three or more independent groups.
Partial least square structural equation modelling (PLS-SEM) was used to predict factors influencing HADS-A, HADS-D, and HADS-T. This technique assesses the reliability of the data set, the statistical significance of the coefficients, and the error of the estimated path coefficients [20]. The bootstrapped significance calculation was performed in SmartPLS software version 3.2.8 (SmartPLS GmbH) [21]. Prior to running the path model, the construct validity (convergent and discriminant) was tested. In the PLS-SEM model, HADS (with two subscales), perceived susceptibility, and severity were considered as reflective constructs, and all other independent variables were single-item constructs. Results of the measurement model indicated that all indicators had an acceptable outer loading (> 0.5) with a CR (composite reliability) value of more than 0.7 and an AVE (average variance extracted) above 0.5. The variance inflation factors (VIFs) for all indicators were below 2.5, which revealed that all indicators belonging to these two constructs were adequately independent. Discriminant validity assessment through the heterotrait-monotrait (HTMT) ratio of correlations method also indicated that all HTMT values were lower than the most restrictive threshold (0.85) proposed by Kline (2011) [22], thereby indicating adequate discriminant validity.
Results
Participants
A total of 631 responses were received and analysed. Participants’ ages ranged from 21 to 86 years (mean = 56.98, SD = 11.21). As shown in Table 1, according to demographics, the majority of the study participants were females (72.4%), of Chinese ethnicity (52.6%). Nearly two thirds were college or university graduates (64.3%), and the highest proportion had incomes of MYR4000–8000. Almost an equal number of participants had been diagnosed with cancer for 4 years and below (40.3%) and between 5 and 9 years (40.6%). The vast majority of the participants were survivors of breast (61.8%) and prostate (25.8%) cancer. The majority were in stage 2 (39.0%) and stage 1 (30.1%) of cancer at the time of first diagnosis. In regard to the treatment received, most of them had undergone surgery (90.5%), followed by chemotherapy (64.0%). Only 31.7% reported having been diagnosed with another chronic disease (or diseases), and 36.9% reported that their health status was fair/poor.
Findings on perceived susceptibility showed that slightly more than half (53.4%) reported that they strongly agreed/agreed that they were worried about getting coronavirus disease. A total of 57.7% reported strongly agreed/agreed that getting the coronavirus disease is possible. A higher proportion reported strongly agreeing/agreeing that they are worried about getting the coronavirus disease (70.0%). In regard to perceived severity, a high proportion reported strongly agreeing/agreeing that complications from the coronavirus disease are serious (95.7%), that they will be very sick if they catch the coronavirus disease (82.9%), and that they are afraid of getting the coronavirus disease (89.5%).
HADS scores by demographics and phases of MCO
The mean HADS-A and HADS-D scores for the study participants were 5.54 (± 3.87) and 4.51 (± 3.70), respectively. The mean HADS-T score was 9.96 (± 7.01). In total, 29.0% (95% CI 25.5–32.7) reported a HADS-A score > 8, and 20.8% (95% CI 17.7–24.1) had a HADS-D score > 8. Of these, 104 participants (16.5%, 95% CI 13.7–17.6) had combined anxiety and depression (both HADS-A and HADS-D scores > 8). A total of 22.3% (95% CI 19.2–25.8) reported a HADS-T score > 16. As shown in Table 1, the mean HADS-A scores were significantly higher in participants with the highest secondary school education or below than those with college or university degrees.
The mean score of the anxiety subscale, HADS-A, was also significantly higher among those with an average household income of MYR4001–8000 than MYR4000 and below and >MYR8000. By ethnicity, the mean HADS-A scores were highest among the Indian and the indigenous groups of Sabah or Sarawak. Mean HADS-D scores increase with increasing age and were significantly higher in males (5.51 ± 3.86) than females (4.01 ± 3.56). Similarly, HADS-D scores were highest among the indigenous groups of Sabah or Sarawak (7.01 ± 4.25), in participants with the highest secondary school education (5.57 ± 4.26), and among those with an average household income of MYR4001–8000 (4.85 ± 3.76). Similar differences were observed in the mean HADS-T scores by demographic characteristics.
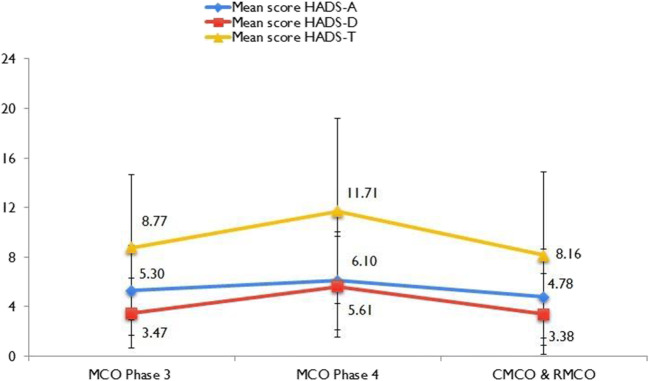
Of overall 631 responses, the proportion responded during the MCO phase 3, MCO phase 4, and CMCO periods were 30.3 (n = 191), 45.5 (n = 287), and 24.2% (153), respectively. Figure 2 shows the distribution of mean HADS-A, HADS-D, and HADS-T scores by phases of MCO. The highest HADS-A (6.10; 95% CI 5.64–6.56), HADS-D (5.61; 95% CI 5.14–6.08), and HADS-T (11.71; 95% CI 10.84–12.58) scores were reported among respondents during phase 3 of the MCO. A reduction in mean scores was observed during the CMCO and RMCO phases. A greater gradient of increase in the trend line slope was observed in HADS-D and HADS-T from phase 3 to phase 4 CMO, and similarly a higher gradient decrease in the trend line was observed from phase 4 MCO to the CMCO/RMCO phases.
Fig. 2.
The trend line of mean HADS-A, HADS-D, and HADS-T scores across the phases of Movement Control Order in Malaysia
HADS scores by cancer types and treatment history
There are no significant differences in the mean HADS-A, HADS-D, and HADS-T scores by time since cancer diagnosis. However, the highest mean HADS-A, HADS-D, and HADS-T scores were recorded among participants who were at stage 4 of cancer at the time of first diagnosis. The mean HADS-A, HADS-D, and HADS-T scores increased as the stage of cancer at the time of first diagnosis increased. Participants who had bladder cancer reported the highest mean HADS-A, HADS-D, and HADS-T scores. Participants who have received chemotherapy reported the highest mean HADS-A (6.12 ± 3.92), HADS-D (4.96 ± 3.96), and HADS-T (11.08 ± 7.26) scores compared with other types of treatment.
HADS scores by chronic diseases and health status
The mean HADS-A, HADS-D, and HADS-T scores were significantly higher in participants who reported having chronic diseases than without chronic diseases. There was a significant increase in the mean HADS-A, HADS-D, and HADS-T scores as the overall health status decreased. The mean HADS-A (10.58 ± 3.84), HADS-D (12.13 ± 3.12), and HADS-T (22.71 ± 5.77) scores exceeded the threshold scores denoted for anxiety, depression, and psychological distress.
HADS by risk perception
As shown in Table 1, all the participants who said they strongly agreed/agreed with all the perceived susceptibility and severity items reported higher mean HADS-A, HADS-D, and HADS-T scores than those who strongly disagreed/disagreed.
Factors predicting HADS on PLS-SEM
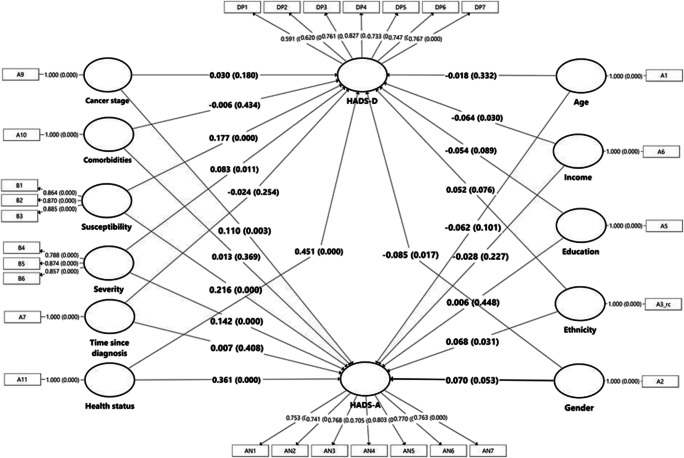
The PLS-SEM in Fig. 3 shows the hypothesized associations for all the factors associated with HADS-A and HADS-D scores. Among all the factors, health status (B = 0.361, p < 0.001), perceived susceptibility (B = 0.216, p < 0.001), and perceived severity (B = 0.142, p < 0.001) have the greatest effect on a higher HADS-A score. Stage of cancer (B = 0.110, p = 0.002) and ethnicity (B = 0.068, p < 0.03) are also significant factors with a smaller effect in predicting a higher HADS-A score. The results for adjusted R2 indicated that this model explained 33.3% of the total variance in HADS-A.
Fig. 3.
The partial least square structural equation modelling (PLS-SEM) model of factors influences HADS-A and HADS-D
For the association between the hypothesized factors and HADS-D, likewise health status (B = 0.451, p < 0.001), perceived susceptibility (B = 0.177, p < 0.001), and severity (B = 0.083, p = 0.011) showed the greatest effect in influencing higher HADS-D scores. Income (B = −0.064, p = 0.033) is inversely associated with a higher HADS-D score, whereas being female (B = −0.085, p = 0.016) is associated with a higher HADS-D. The results for adjusted R2 indicated that this model explained 41.4% of the total variance in HADS-D.
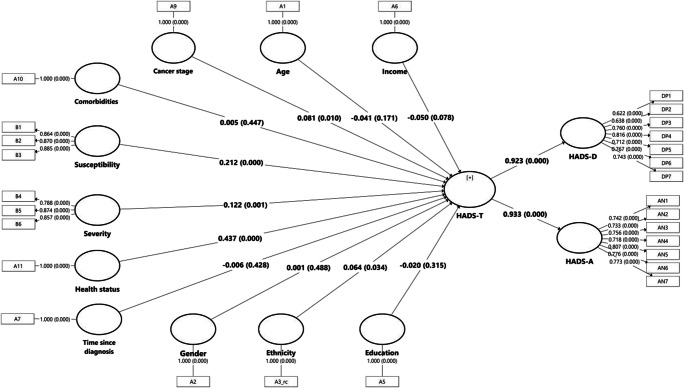
The PLS-SEM in Fig. 4 shows the hypothesized associations of all the factors associated with HADS-T. As depicted in the figure, health status (B = 0.437, p < 0.001), perceived susceptibility (B = 0.212, p < 0.001), and severity (B = 0.122, p = 0.001) showed the greatest effect in influencing a higher HADS-T score. A higher cancer stage (B = 0.081, p = 0.011) is associated with a higher HADS-T score. Ethnicity (B = 0.064, p = 0.033) shows a weak and significant association with HADS-T. The results for adjusted R2 indicated that this model explained 41.1% of the total variance in HADS-A.
Fig. 4.
The partial least square structural equation modelling (PLS-SEM) model of factors influences HADS-T
Discussion
COVID-19 is an emerging infectious disease that poses a significant threat to people who have survived cancer. Given the serious threats imposed by COVID-19, people who have survived cancer face a time of unprecedented fear and anxiety that may increase an existing threat. This study assesses psychological distress among cancer survivors, and responses were captured through phases 3 and 4 of the MCO, throughout the entire CMCO, and in the early phase of the RMCO. Understanding the extent to which cancer survivors show psychological impairment during the COVID-19 pandemic is important in preventing it or treating its consequences.
The HADS-A score demonstrated that 29.0% of the study participants had anxiety, whereas a slightly lower proportion (20.9%) demonstrated depression symptom based on HADS-D scores. The proportion of anxiety and depression symptoms found among the participants in this study is higher than that reported in a recent study on cancer patients in the USA, which indicated that a total of 23.3% demonstrated HADS-A and only 7.6% reported HADS-D above the threshold score of 8 during the COVID-19 pandemic [23]. The low psychological impact among cancer patients in the US study was reported as probably being due to the fact that a high proportion of them are users of the counselling (56%) and networking services (80%) provided [23]. Also, the study was conducted at a time when the number of local confirmed cases in the USA was low. It is also important to highlight that based on the HADS-T score, a total of 22.3% demonstrated psychological distress in this study. To our best knowledge, no study has measured psychological distress on cancer patients and survivors using HADS-T during the COVID-19 pandemic; however, distress assessed by event scale–revised (IES-R) found 20.8% of breast cancer patients reported severe distress sequels during the outbreak in China [24]. In France, psychological distress measured using psychological distress inventory (PDI) revealed 34% of breast cancer patients reported depressive disorder [25]. The outbreak of COVID-19 may disproportionately affect cancer survivors by exacerbating their prior traumatic cancer experience and worsening mental health distress [26]. Nonetheless, collective evidence from this study and the above-mentioned studies suggests that screening for patients’ levels of psychological distress is essential. Previous studies reported that both survivors and cancer patients should be provided with guidance and counselling on psychological adaptation during the pandemic period [23, 27]. In Malaysia, a helpline providing counselling services to support people experiencing emotional distress during the COVID-19 pandemic is available [28]; however, the counselling services are for the general public and not specifically for cancer patients or survivors. This current study unfortunately did not gather information about the use of counselling service among our study participants.
The findings of this study showed an increase in the trends of anxiety, depression, and psychological distress across the different phases of the MCO as the COVID-19 pandemic progresses in Malaysia. The MCO, first imposed on 18 March, was extended until 9 June 2020. The four phases of the MCO have lasted 14 days each. Religious, sports, social, and cultural activity restrictions, social distancing and a loss of social cohesion, interstate travel bans, travel restrictions, and financial hardship due to job loss or reduced income associated with the shutdown of many industries during the implementation of the MCO have resulted in an increasing psychological impact across the four phases of the MCO. The extensions and prolonged MCO period have resulted in high psychological distress among the participants in the four phases of the MCO in this study. However, in the CMCO and RMCO phases, a decrease in the trend line of anxiety, depression, and psychological distress was observed when some restrictions were lifted and businesses reopened. It is worth noting that our results revealed that the greater gradient in the increase and decrease of the depression trend line compared to anxiety may imply that cancer survivors were more at risk of depression disorder during the MCO period. The findings of increasing psychological consequences as the pandemic progress imply the growing need for continuous provision of counselling and mental health assistance for cancer survivors.
Previous findings on the association of anxiety and depression with age in cancer patients have been mixed. In a study on a geriatric oncology population, while increased age was significantly associated with reduced anxiety, it was also associated with greater depressive symptoms [29]. There has also been a report that as age increased, anxiety decreased significantly; however, age was not a significant predictor of depression [30]. A study on anxiety and depression in working-age cancer survivors reported that higher anxiety was associated with younger age; nonetheless, no significant association between depression and age was found [31]. In our study, there was no significant difference between anxiety and age; nevertheless, significant increases in depression and psychological distress were reported as age increased in the univariate analyses. However, in the PLS-SEM, age is not a significant predictor of anxiety, depression, or psychological distress. It is well documented that females are more likely than males to develop anxiety disorder [32]. Most studies on the general public reported higher anxiety and psychological impact in females than males during the COVID-19 pandemic [33–35], including a study in Malaysia [36]. Likewise, in this study, while gender is not a significant predictor of anxiety, the structural model indicated that being female is significantly associated with higher depression. Ethnicity, on the other hand, is a significant predictor of anxiety but not depression. It is worth noting that in the study, the Chinese reported the lowest anxiety and depression scores, whereas the highest scores were reported among the Indian and the indigenous groups from Sabah or Sarawak. In this study, the level of educational attainment is not a significant predictor of anxiety, depression, or psychological distress in all three structural equation modellings; nevertheless, in the univariate analyses, the scores for anxiety, depression, and psychological distress were significantly higher among survivors of lower educational attainment. A previous study reported that a higher educational level seems to have a protective effect against anxiety and depression across the lifespan [37]. This study also found that a low level of income was significant and associated with depression in the structural equation modelling, likewise found in other studies. [38, 39]. The findings of this study provide insights into the demographics of cancer survivors who are at higher risk of mental illness to effectively target the provision of behavioural counselling intervention.
The stage of cancer at diagnosis and comorbidities (both before and after the cancer diagnosis) have been reported to affect the mental health and quality of life of cancer survivors [12]. Similarly, the findings of this study show that during the COVID-19 pandemic, the stage of cancer at diagnosis was significantly associated with anxiety and psychological distress but not depression. In regard to the influence of comorbidities, our study revealed that comorbidities were not associated with anxiety, depression, or psychological distress. Overall perceived health is recognized as a powerful predictor of negative health outcomes and low health-related quality of life [40, 41]. The PLS-SEM path analyses in this study revealed that overall perceived health was the strongest predictor of anxiety, depression, and psychological distress. Our findings suggest that interventions that aim to adjust patients’ illness perceptions to facilitate a reduction of psychological distress during the COVID-19 pandemic are needed. As self-perceived health status is the assessment of narrative belief, there is also a need to further investigate cancer survivors’ experiences and the actual physical health conditions underlying their self-reported health perceptions. As noted earlier, comorbidities, which were also self-reported, were found to be not associated with anxiety, depression, or psychological distress.
The widespread media coverage and reports concerning older people and people who have underlying medical conditions being at increased risk of severe illness from COVID-19 have perhaps led to many of the cancer survivors in this study reporting a high-risk perception of COVID-19. We also found a particularly strong positive association between risk perception and anxiety, depression, and psychological distress. The participants with more intense perceived susceptibility and severity showing higher psychological distress found in this study were similar to those reported in a previous study on the Malaysian general population, where risk perception was found to enhance anxiety as measured by the State-Trait Anxiety Inventory (STAI) [36]. Hence, the findings imply the need to establish proper and effective risk communication surrounding COVID-19 to avoid causing psychological distress among cancer survivors during the pandemic.
This survey is believed to provide snapshots of the levels as well as the correlates of psychological and mental health of cancer survivors in the era of COVID-19. The evidence of mental distress and identification of distress correlates suggests intervention targets. As there is currently little support available for cancer survivor community, this study potentially provides powerful insights for healthcare providers to develop appropriate support to address cancer survivors’ needs during the pandemic of infectious disease. Our study presents some evident limitations, which may have influenced the reporting of the results. Firstly, the key disadvantage of a cross-sectional online survey is that our sample may lack generalizability of the cancer survivor population in Malaysia. Nonetheless, our participant sample population consisted of well-distributed diversity in terms of demographics and illness conditions. Second, the responses were based on self-reports and may be subject to recall bias, self-reporting bias, and a tendency to report socially desirable responses. It is also important to note that the survey was conducted after the peak of COVID-19 in Malaysia. The study findings, therefore, have to be interpreted with caution.
Conclusion
The mental health of cancer survivors warrants the provision of psychological support during the COVID-19 pandemic. In particular, we highlight that during quarantine or restriction of movement enforcement, psychological distress among cancer survivors increased along with the increased duration of the restriction. Hence, findings suggest that there is an essential need for cancer survivors or their carers to regularly monitor their psychological status during infectious disease pandemic and to facilitate timely intervention. Appropriate risk perception communication is warranted for people who are immunocompromised, such as cancer survivors, in order not to cause unnecessary panic. Individual characteristics such as socio-economic and comorbid health conditions, and characteristics of cancer that are associated with psychological distress found in this study, offer insights into high-risk cancer survivors in terms of mental health screening, monitoring, and support.
Acknowledgements
The authors would like to thank Mrs Lim Chiou Ling, chairwoman of Candy Girls Breast Cancer Support Group University Malaya Medical Centre; Ms Ranjit Kaur, chairman of Breast Cancer Welfare Association; Mr Peter Wong, chairperson of Exercise Support Group University Malaya Medical Centre; ROSE Foundation; Prof Dr. Ong Teng Aik; Prof Dr. Woo Yin Ling; and all cancer survivors for their contributions in the research project.
Author contribution
All authors contributed to the study conception and design. Material preparation and analysis were performed by Li Ping Wong, Haridah Alias, and Mahmoud Danaee. Data collection was performed by Lai Lee Lee, See Mee Hoong, Peter Seah Keng Tok, and Ting Chuo Yew. The first draft of the manuscript was written by Li Ping Wong, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
This study was approved by the University of Malaya Research Ethics Committee (UMREC), approval code: UM.TNC2/UMREC – 885
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO (2020) Coronavirus disease 2019 (COVID-19) situation report – 71. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200331-sitrep-71-covid-19.pdf?sfvrsn=4360e92b_8. Accessed 20 July 2020
- 4.Swainston J, Chapman B, Grunfeld EA, Derakshan N. COVID-19 lockdown and its adverse impact on psychological health in breast cancer. Front Psychol. 2020;11:2033. doi: 10.3389/fpsyg.2020.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society (2020) COVID-19 Pandemic impact on cancer patients and survivors: survey findings summary. https://www.fightcancer.org/releases/survey-covid-19-affecting-patients%E2%80%99-access-cancer-care-13. Accessed 2 January 2021
- 6.Tsamakis K, Gavriatopoulou M, Schizas D, Stravodimou A, Mougkou A, Tsiptsios D, Sioulas V, Spartalis E, Sioulas AD, Tsamakis C, Charalampakis N. Oncology during the COVID-19 pandemic: challenges, dilemmas and the psychosocial impact on cancer patients. Oncol Lett. 2020;20:441–447. doi: 10.3892/ol.2020.11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Quteimat OM, Amer AM. The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol. 2020;43:452–455. doi: 10.1097/COC.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeoh CB, Lee KJ, Rieth EF, Mapes R, Tchoudovskaia AV, Fischer GW, Tollinche LE. COVID-19 in the cancer patient. Anesth Analg. 2020;131:16–23. doi: 10.1213/ANE.0000000000004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romeo A, Di Tella M, Ghiggia A, Tesio V, Torta R, Castelli L Posttraumatic growth in breast cancer survivors: are depressive symptoms really negative predictors? Psychol Trauma-US 12:244. 10.1037/tra0000508 [DOI] [PubMed]
- 11.National Cancer Institute (2016) The Malaysian National Cancer Registry Report (MNCR) 2007-2011. https://www.crc.gov.my/wp-content/uploads/documents/report/MNCRRrepor2007-2011.pdf. Accesed 20 July 2020
- 12.Naughton MJ, Weaver KE. Physical and mental health among cancer survivors: considerations for long-term care and quality of life. N C Med. 2014;75:283–286. doi: 10.18043/ncm.75.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niedzwiedz CL, Knifton L, Robb KA, Katikireddi SV, Smith DJ. Depression and anxiety among people living with and beyond cancer: a growing clinical and research priority. BMC Cancer. 2019;19:1–8. doi: 10.1186/s12885-019-6181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dryhurst S, Schneider CR, Kerr J, Freeman AL, Recchia G, Van Der Bles AM, Spiegelhalter D, van der Linden S (2020) Risk perceptions of COVID-19 around the world. J Risk Res:1–3. 10.1080/13669877.2020.1758193
- 15.Becker MH. The health belief model and personal health behavior. Health Educ Monogr. 1974;19:324–508. [Google Scholar]
- 16.Rosenstock IM. Historical origins of the health belief model. Health Educ Monogr. 1974;2:328–335. doi: 10.1177/109019817400200403. [DOI] [Google Scholar]
- 17.Champion V, Skinner CS. The Health Belief Model. In: Glanz K, Rimer B, Viswanath K, editors. Health behavior and health education. 4. San Francisco: Jossey-Bass; 2008. pp. 45–65. [Google Scholar]
- 18.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 19.Vodermaier A, Millman RD. Accuracy of the hospital anxiety and depression scale as a screening tool in cancer patients: a systematic review and meta-analysis. Support Care Cancer. 2011;19:1899–1908. doi: 10.1007/s00520-011-1251-4. [DOI] [PubMed] [Google Scholar]
- 20.Chin WW. The partial least squares approach to structural equation modeling. Mod Methods Bus Res. 1998;295:295–336. [Google Scholar]
- 21.Ringle CM, Wende S, Becker JM. SmartPLS 3. Boenningstedt: SmartPLS GmbH; 2015. [Google Scholar]
- 22.Kline RB. Principles and practice of structural equation modeling. New York: Guilford Press; 2011. [Google Scholar]
- 23.Frey MK, Ellis AE, Zeligs K, Chapman-Davis E, Thomas C, Christos PJ, Kolev V, Prasad-Hayes M, Cohen S, Holcomb K, Blank SV. Impact of the COVID-19 pandemic on quality of life for women with ovarian cancer. Am J Obstet Gynecol. 2020;223:725.e1–725.e9. doi: 10.1016/j.ajog.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juanjuan L, Santa-Maria CA, Hongfang F, Lingcheng W, Pengcheng Z, Yuangbing X, Yuyan T, Zhongchun L, Bo D, Meng L, Qingfeng Y. Patient reported outcomes of breast cancer patients during the COVID-19 outbreak in the epicenter of China: a cross sectional survey study. Clin Breast Cancer. 2020;20:e651–e662. doi: 10.1016/j.clbc.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaix B, Delamon G, Guillemasse A, Brouard B, Bibault JE (2020) Psychological distress during the COVID-19 pandemic in France: a national assessment of at-risk populations. BMJ 33. 10.1136/gpsych-2020-100349 [DOI] [PMC free article] [PubMed]
- 26.Nekhlyudov L, Duijts S, Hudson SV, Jones JM, Keogh J, Love B, Lustberg M, Smith KC, Tevaarwerk A, Yu X, Feuerstein M. Addressing the needs of cancer survivors during the COVID-19 pandemic. J Cancer Surviv. 2020;14:601–606. doi: 10.1007/s11764-020-00884-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spicer J, Chamberlain C, Papa S. Provision of cancer care during the COVID-19 pandemic. Nat Rev Clin Oncol. 2020;17:1–3. doi: 10.1038/s41571-020-0370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mercy Malaysia (2020) Sokongan Psikososial Covid-19. https://www.mercy.org.my/popupbuilder/covid-19-hotline/mercy-malaysia-sokongan-psikososial-covid-19/. Accessed 20 July 2020
- 29.Nelson CJ, Weinberger MI, Balk E, Holland J, Breitbart W, Roth AJ. The chronology of distress, anxiety, and depression in older prostate cancer patients. Oncologist. 2009;14:891–899. doi: 10.1634/theoncologist.2009-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss Wiesel TR, Nelson CJ, Tew WP, Hardt M, Mohile SG, Owusu C, Klepin HD, Gross CP, Gajra A, Lichtman SM, Ramani R. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24:712–717. doi: 10.1002/pon.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inhestern L, Beierlein V, Bultmann JC, Möller B, Romer G, Koch U, Bergelt C. Anxiety and depression in working-age cancer survivors: a register-based study. BMC Cancer. 2017;17:1–8. doi: 10.1186/s12885-017-3347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blbas HT, Aziz KF, Nejad SH, Barzinjy AA (2020) Phenomenon of depression and anxiety related to precautions for prevention among population during the outbreak of COVID-19 in Kurdistan Region of Iraq: based on questionnaire survey. J Public Health (Oxf):1–5. 10.1007/s10389-020-01325-9 [DOI] [PMC free article] [PubMed]
- 34.Guo X, Meng Z, Huang G, Fan J, Zhou W, Ling W, Jiang J, Long J, Su L. Meta-analysis of the prevalence of anxiety disorders in mainland China from 2000 to 2015. Sci Res. 2016;6:1–5. doi: 10.1038/srep28033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varshney M, Parel JT, Raizada N, Sarin SK. Initial psychological impact of COVID-19 and its correlates in Indian Community: An online (FEEL-COVID) survey. PLoS One. 2020;15:e0233874. doi: 10.1371/journal.pone.0233874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong LP, Alias H (2020) Temporal changes in psychobehavioural responses during the early phase of the COVID-19 pandemic in Malaysia. J Behav Med. 10.21203/rs.3.rs-24316/v1 [DOI] [PMC free article] [PubMed]
- 37.Bjelland I, Krokstad S, Mykletun A, Dahl AA, Tell GS, Tambs K. Does a higher educational level protect against anxiety and depression? The HUNT study. Soc Sci Med. 2008;66:1334–1345. doi: 10.1016/j.socscimed.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 38.Sareen J, Afifi TO. McMillan KA, Asmundson GJ. Relationship between household income and mental disorders: findings from a population-based longitudinal study. Arch Gen Psychiatry. 2011;68:419–427. doi: 10.1001/archgenpsychiatry.2011.15. [DOI] [PubMed] [Google Scholar]
- 39.Akhtar-Danesh N, Landeen J. Relation between depression and sociodemographic factors. Int J Ment Heal Syst. 2020;1:4. doi: 10.1186/1752-4458-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beverly C, Pozehl B, Hertzog M, Zimmerman L, Riegel B. Predictors of overall perceived health in patients with heart failure. J Cardiovasc Nurs. 2013;28:206–215. doi: 10.1097/JCN.0b013e31824987a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.