Supplemental Digital Content is available in the text.
Keywords: osteoporosis, osteosarcopenia, primary biliary cholangitis, sarcopenia, vertebral fracture
Abstract
Aim
Bone disorders are serious complications in patients with primary biliary cholangitis (PBC), especially in postmenopausal female patients. Given that osteoporosis interrelates closely with sarcopenia, the concept of osteosarcopenia (coexistence of the two complications) has been established. This study aimed to investigate the relationship between osteoporosis, sarcopenia, vertebral fracture, and osteosarcopenia in PBC patients.
Methods
This study involved 117 consecutive PBC patients (21 males and 96 females). Bone mineral density (BMD) was measured with dual-energy X-ray absorptiometry. Sarcopenia was diagnosed according to the Japan Society of Hepatology assessment criteria.
Results
Of the 117 patients, 33 (28.2%), 27 (23.1%), 21 (17.9%), and 18 (15.4%) had osteoporosis, sarcopenia, vertebral fracture, and osteosarcopenia, respectively. Multivariate analysis identified sarcopenia as a significant, independent risk factor associated with osteoporosis in all and female patients [odds ratio (OR) = 4.126, P = 0.018; OR = 6.510, P = 0.001, respectively], and vice versa (OR = 3.420, P = 0.040; OR = 4.012, P = 0.026, respectively). The skeletal muscle mass index and handgrip strength were significantly correlated with the BMD of the lumbar spine, femoral neck, and total hip (r = 0.46–0.59, P < 0.001). Patients with osteosarcopenia had significantly higher prevalence of vertebral fracture (10/18; 55.6%) than those without both osteoporosis and sarcopenia (5/75; 6.7%).
Conclusion
We demonstrated the prevalence of osteoporosis, sarcopenia, vertebral fracture, and osteosarcopenia in PBC, and noted that these complications interrelated closely with each other. Comprehensive assessment and treatment strategies for bone and muscle disorders are essential for PBC patients.
Introduction
Primary biliary cholangitis (PBC) is a chronic autoimmune-mediated cholestatic liver disease occurring predominantly in middle-aged women and eventually progressing to liver failure [1]. Given that PBC occurs nine times more frequently in women than in men, sex-related factors could affect its pathogenesis and development [2]. PBC is occasionally complicated by osteoporosis due to malabsorption of calcium and vitamin D. Furthermore, hyperbilirubinemia, which develops more frequently and severely with progression of disease than other liver diseases, impairs osteoblast function, resulting in promotion of osteoporosis. These disease conditions predispose patients to fragility fractures affecting morbidity and health-related quality of life [3–9].
The reported prevalence of osteoporosis among PBC patients varies from 20 to 37% and is higher than that in the general population (10–11%) [3–8]. Specifically, osteoporosis is closely linked to menopause in women and advanced disease stages [3,4,10,11]. Menopause causes a drastic decline in estrogen, leading to a decrease in bone mass (osteoporosis), and muscle mass and strength (sarcopenia) [12,13]. These findings and fact mean that reduced sex hormone concurrent with PBC may increase the frequency and enhance the severity of musculoskeletal disorders. Therefore, declining physical function and health-related quality of life due to osteoporosis and sarcopenia are critical concerns in postmenopausal female patients with PBC.
Sarcopenia is characterized by the loss of skeletal muscle mass and strength, and classified into primary sarcopenia when muscle loss is caused by aging and secondary sarcopenia where the loss is caused by underlying diseases, such as chronic liver disease (CLD), inflammatory disorder, and malignancy [14]. Specifically, secondary sarcopenia caused by CLD has become a clinical focus, given that it aggravates health-related quality of life and prognoses for patients with CLD, including those with PBC [15–17]. Hence, appropriate assessment and treatment of sarcopenia are imperative for PBC patients. Of note, sarcopenia and osteoporosis closely interrelate with each other and frequently coexist in the elderly general population and CLD patients [18–22]. Consequently, the term ‘osteosarcopenia’ has been recently defined as the coexistence of sarcopenia and osteoporosis [22–25]. The presence of osteosarcopenia exacerbates negative health outcomes and has been described as a ‘hazardous duet’, because it causes both ease of falling (due to sarcopenia) and bone vulnerability (due to osteoporosis) [25,26].
Despite the clinical research advancements in bone disorders, the accurate prevalence and influencing factors for sarcopenia and osteosarcopenia in PBC patients have not been fully elucidated. Therefore, the aim of this study was to assess the factors associated with osteoporosis, sarcopenia, and osteosarcopenia and clarify the relationship among these complications in PBC patients.
Methods
Study design and patients
This cross-sectional study involved 117 consecutive Japanese patients diagnosed with PBC at the Jikei University School of Medicine (Tokyo, Japan) and Fuji City General Hospital (Shizuoka, Japan) between 2017 and 2019. All patients had been receiving ursodeoxycholic acid (UDCA) or combination of UDCA plus bezafibrate for the treatment of PBC at study entry. Patients were diagnosed with PBC if they satisfied any one of the following criteria: (1) biochemical evidence of cholestasis and histological evidence of chronic non-suppurative destructive cholangitis; (2) presence of anti-mitochondrial antibody (AMA) with histologically compatible features of PBC; and (3) presence of AMA with clinical features and course characteristic of PBC, regardless of histological findings [27]. The inclusion criteria were as follows: (1) patients diagnosed with PBC; (2) measurement of skeletal muscle mass index (SMI) with a bioelectrical impedance analysis (InBody S10; InBody, Seoul, Korea); (3) measurement of handgrip strength with a grip dynamometer (T.K.K5401 GRIP-D; Takei Scientific Instruments, Niigata, Japan); (4) measurement of bone mineral density (BMD) with a dual-energy X-ray absorptiometry (PRODIGY; GE Healthcare, Madison, Wisconsin, USA); and (5) evaluation of vertebral fracture using spinal lateral X-rays. Patients with coexisting other liver diseases (such as hepatitis B/C, alcohol liver disease, and nonalcoholic steatohepatitis) were excluded. This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Jikei University School of Medicine (approval no. 28-196) and Fuji City General Hospital (approval no. 162). Written informed consent was obtained from all patients.
Diagnosis of sarcopenia and osteoporosis
The diagnosis of sarcopenia was based on the guidelines proposed by the Japan Society of Hepatology [15]. Briefly, sarcopenia was defined as low handgrip strength and low muscle mass. The cutoff values of low handgrip-strength diagnosis were 26 kg for men and 18 kg for women. The SMI was calculated as the sum of the muscle mass of four limbs, divided by height squared (kg/m2). The SMI cutoff values for low muscle-mass diagnosis were 7.0 kg/m2 for men and 5.7 kg/m2 for women. BMD was assessed at the lumbar spine (L2–L4), femoral neck, and total hip. The diagnosis of osteoporosis was based on the WHO criteria (T-score ≤ −2.5 for osteoporosis, −2.5 to −1.0 for osteopenia, and >−1.0 for normal) [28]. Vertebral fracture was evaluated using spinal lateral X-rays.
Clinical and laboratory assessment
Blood samples were obtained from each patient after overnight fasting. Serum aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyltransferase, total bilirubin, albumin, Mac-2 binding protein glycosylation isomer (M2BPGi; hepatic fibrosis marker), insulin-like growth factor 1 (IGF-1), branched-chain amino acid (BCAA), tartrate-resistant acid phosphatase-5b, and total procollagen type I N-terminal propeptide were determined by routine laboratory methods.
Statistical analysis
Continuous variables were represented as medians and interquartile ranges in parentheses. The Mann–Whitney U test and the Kruskal–Wallis test were used to evaluate the differences in the distribution of continuous variables between two groups and among the four groups, respectively. Categorical variables were represented as the number of patients and percentages in parentheses. The chi-squared test was used to evaluate the group differences in categorical variables. Univariate analysis was performed initially to identify possible variables that achieved P < 0.1 and significantly related to sarcopenia and osteoporosis. Subsequently, multiple logistic regression analysis was performed to identify significant variables independently associated with sarcopenia and osteoporosis. Correlations between two continuous variables were analyzed using the Spearman’s rank correlation test. Statistical analyses were performed using SPSS version 26 software (IBM, Armonk, New York, USA). A P-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
The baseline clinical characteristics of the 117 patients enrolled in this study are shown in Table 1. The patient cohort comprised 21 males (17.9%) and 96 females (82.1%). The median ages at entry and PBC diagnosis were 68.0 (56.5–73.0) years and 61.0 (51.0–69.0) years, respectively. The median SMI and handgrip strength were 6.09 (5.54–6.80) kg/m2 and 22.5 (17.6–26.0) kg/m2, respectively. The median BMD values of the lumber spine, femoral neck, and total hip were 1.06 (0.87–1.19) g/cm2, 0.75 (0.67–0.88) g/cm2, and 0.81 (0.72–0.89) g/cm2, respectively. The prevalence of vertebral fracture was 17.9% (21/117).
Table 1.
Comparison of clinical characteristics between patients with and without osteoporosis
| Variable | All patients | Osteoporosis | Non-osteoporosis | P-value |
|---|---|---|---|---|
| Patients, n (%) | 117 | 33 (28.2) | 84 (71.8) | |
| Female, n (%) | 96 (82.1) | 29 (87.9) | 67 (79.8) | 0.505 |
| Age (years) | 68.0 (56.5–73.0) | 73.0 (67.5–78.5) | 63.0 (54.2–71.0) | <0.001 |
| BMI (kg/m2) | 22.3 (20.4–24.9) | 20.4 (18.6–22.7) | 22.9 (20.9–25.9) | <0.001 |
| Liver cirrhosis, n (%) | 11 (9.4) | 6 (18.2) | 5 (6.0) | 0.059 |
| Age at diagnosis (years) | 61.0 (51.0–69.0) | 66.5 (56.3–72.8) | 57.0 (49.0–67.0) | 0.003 |
| Duration of disease (years) | 2.0 (1.0–7.0) | 4.0 (1.3–11.3) | 2.0 (1.0–6.0) | 0.042 |
| AST (IU/L) | 25 (20–31) | 26 (23–30) | 24 (14–27) | 0.343 |
| ALT (IU/L) | 20 (14–24) | 18 (14–22) | 20 (14–27) | 0.453 |
| ALP (IU/L) | 283 (225–347) | 271 (211–323) | 291 (230–360) | 0.268 |
| GGT (IU/L) | 39 (26–67) | 32 (25–57) | 43 (28–72) | 0.337 |
| Total bilirubin (mg/dl) | 0.6 (0.5–0.8) | 0.5 (0.4–0.7) | 0.6 (0.5–0.8) | 0.130 |
| Albumin (g/dl) | 4.1 (3.9–4.3) | 4.0 (3.8–4.2) | 4.2 (3.9–4.3) | 0.021 |
| M2BPGi (C.O.I) | 0.87 (0.60–1.31) | 1.16 (0.67–1.63) | 0.80 (0.57–1.17) | 0.018 |
| IGF-1 (ng/ml) | 90 (61–113) | 61 (46–101) | 95 (72–117) | 0.002 |
| BCAA (μmol/L) | 419 (361–477) | 375 (318–430) | 441 (386–500) | 0.001 |
| TRACP-5b (mU/dl) | 346 (245–448) | 388 (281–458) | 336 (273–441) | 0.323 |
| P1NP (ng/ml) | 47 (34–59) | 47 (34–64) | 45 (34–58) | 0.909 |
| SMI (kg/m2) | 6.09 (5.54–6.80) | 5.39 (4.97–5.93) | 6.39 (5.90–7.08) | <0.001 |
| Handgrip strength (kg) | 22.5 (17.6–26.0) | 17.3 (14.7–22.0) | 23.5 (20.1–27.0) | <0.001 |
| Lumbar spine BMD (g/cm2) | 1.06 (0.87–1.19) | 0.83 (0.73–0.93) | 1.09 (0.98–1.22) | 0.001 |
| Femoral neck BMD (g/cm2) | 0.75 (0.67–0.88) | 0.63 (0.58–0.67) | 0.81 (0.73–0.90) | 0.001 |
| Total hip BMD (g/cm2) | 0.81 (0.72–0.89) | 0.67 (0.60–0.71) | 0.85 (0.78–0.93) | 0.001 |
| Sarcopenia, n (%) | 27 (23.1) | 18 (54.5) | 9 (10.7) | <0.001 |
| Vertebral fracture, n (%) | 21 (17.9) | 13 (39.4) | 8 (9.5) | <0.001 |
Values are shown as median (interquartile range) or number (percentage). Statistical analysis was performed using the chi-squared test or the Mann–Whitney U test, as appropriate
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCAA, branched-chain amino acids; BMD, bone mineral density; GGT, gamma-glutamyltransferase; IGF-1, insulin-like growth factor 1; M2BPGi, Mac-2 binding protein glycosylation isomer; P1NP, procollagen type I N-terminal propeptide; SMI, skeletal muscle mass index; TRACP-5b, tartrate-resistant acid phosphatase 5b.
Comparison of clinical characteristics between patients with and without osteoporosis
Among the 117 patients, 33 (28.2%) were diagnosed with osteoporosis (Table 1). Osteoporosis patients were older (P < 0.001) and had lower BMI (P < 0.001) than non-osteoporosis patients. Patient age at PBC diagnosis was also higher in the osteoporosis group (P = 0.003).
Regarding biochemical parameters, the osteoporosis group had significantly lower levels of albumin (P = 0.021), IGF-1 (P = 0.002) and BCAA (P = 0.001), and higher levels of M2BPGi (P = 0.018) than the non-osteoporosis group. The SMI and handgrip strength values in the osteoporosis group were significantly lower than those in the non-osteoporosis group (P < 0.001 for both). Of note, the prevalence of sarcopenia and vertebral fracture was significantly higher in the osteoporosis group than in the non-osteoporosis group [54.5% (18/33) versus 10.7% (9/84), P < 0.001; and 39.4% (13/33) versus 9.5% (8/84), P < 0.001, respectively].
Comparison of clinical characteristics between patients with and without sarcopenia
Among the 117 patients, 27 (23.1%) were diagnosed with sarcopenia (Table 2). Sarcopenia patients were older (P = 0.006) and had lower BMI (P < 0.001) than non-sarcopenia patients. Similar to osteoporosis, the age at PBC diagnosis was significantly higher in the sarcopenia group (P = 0.010). The sarcopenia group had significantly lower levels of albumin (P = 0.014) and IGF-1 (P = 0.025), and higher levels of M2BPGi (P = 0.001) than the non-sarcopenia group. BMD values of the lumbar spine, femoral neck, and total hip in the sarcopenia group were significantly lower than those in the non-sarcopenia group (P = 0.001 for all). Notably, the prevalence of osteoporosis and vertebral fracture in the sarcopenia group was significantly higher than that in the non-sarcopenia group [66.7% (18/27) versus 16.7% (15/90), P < 0.001; and 48.1% (13/27) versus 8.9% (8/90), P < 0.001, respectively].
Table 2.
Comparison of clinical characteristics between patients with and without sarcopenia
| Variable | Sarcopenia | Non-sarcopenia | P-value |
|---|---|---|---|
| Patients, n (%) | 27 (23.1) | 90 (76.9) | |
| Female, n (%) | 24 (88.9) | 72 (80.0) | 0.291 |
| Age (years) | 73.0 (65.0–77.0) | 66.0 (56.0–71.2) | 0.006 |
| BMI (kg/m2) | 20.1 (18.1–22.2) | 22.8 (20.9–25.9) | <0.001 |
| Liver cirrhosis, n (%) | 6 (22.2) | 5 (5.6) | 0.009 |
| Age at diagnosis (years) | 67.0 (53.0–74.0) | 58.5 (50.0–67.0) | 0.010 |
| Duration of disease (years) | 3.0 (1.0–8.0) | 2.0 (1.0–7.0) | 0.929 |
| AST (IU/L) | 24 (22–33) | 25 (20–30) | 0.798 |
| ALT (IU/L) | 18 (14–21) | 20 (14–26) | 0.227 |
| ALP (IU/L) | 285 (223–337) | 282 (226–351) | 0.951 |
| GGT (IU/L) | 35 (22–68) | 41 (26–67) | 0.477 |
| Total bilirubin (mg/dl) | 0.5 (0.4–0.7) | 0.6 (0.5–0.8) | 0.100 |
| Albumin (g/dl) | 3.9 (3.6–4.2) | 4.2 (3.9–4.3) | 0.014 |
| M2BPGi (C.O.I) | 1.27 (0.73–1.83) | 0.82 (0.56–1.17) | 0.001 |
| IGF-1 (ng/ml) | 72 (47–105) | 93 (67–116) | 0.025 |
| BCAA (μmol/L) | 375 (311–463) | 433 (379–484) | 0.058 |
| TRACP-5b (mU/dl) | 392 (236–480) | 342 (245–431) | 0.467 |
| P1NP (ng/ml) | 41 (32–72) | 47 (35–58) | 0.796 |
| SMI (kg/m2) | 5.12 (4.84–5.30) | 6.37 (5.97–7.07) | <0.001 |
| Handgrip strength (kg) | 16.7 (14.1–17.4) | 23.5 (21.5–26.9) | <0.001 |
| Lumbar spine BMD (g/cm2) | 0.86 (0.75–0.96) | 1.09 (0.94–1.22) | 0.001 |
| Femoral neck BMD (g/cm2) | 0.67 (0.62–0.76) | 0.78 (0.70–0.89) | 0.001 |
| Total hip BMD (g/cm2) | 0.72 (0.65–0.78) | 0.84 (0.76–0.92) | 0.001 |
| Osteoporosis, n (%) | 18 (66.7) | 15 (16.7) | <0.001 |
| Vertebral fracture, n (%) | 13 (48.1) | 8 (8.9) | <0.001 |
Values are shown as median (interquartile range) or number (percentage). Statistical analysis was performed using the chi-squared test or the Mann–Whitney U test, as appropriate.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCAA, branched-chain amino acids; BMD, bone mineral density; GGT, gamma-glutamyltransferase; IGF-1, insulin-like growth factor 1; M2BPGi, Mac-2 binding protein glycosylation isomer; P1NP, procollagen type I N-terminal propeptide; SMI, skeletal muscle mass index; TRACP-5b, tartrate-resistant acid phosphatase 5b.
Factors associated with osteoporosis and sarcopenia, and the relationship between the two complications
The following ten variables were significantly related to osteoporosis in the univariate analysis: ages at entry and PBC diagnosis, BMI, presence of liver cirrhosis, duration of disease, albumin, IGF-1, BCAA, sarcopenia, and vertebral fracture (Table S1, Supplemental digital content 1, http://links.lww.com/EJGH/A544). In the multivariate analysis, the following three variables were significantly and independently associated with osteoporosis: older age [odds ratio (OR) = 1.090, 95% confidence interval (CI) = 1.034–1.148, P = 0.001], lower BMI (OR = 0.823, 95% CI = 0.690–0.982, P = 0.031), and presence of sarcopenia (OR = 4.126, 95% CI = 1.280–13.297, P = 0.018) (Table S1, Supplemental digital content 1, http://links.lww.com/EJGH/A544).
The following nine variables were significantly related to sarcopenia in the univariate analysis: ages at entry and PBC diagnosis, BMI, presence of liver cirrhosis, albumin, M2BPGi, IGF-1, osteoporosis, and vertebral fracture (Table S2, Supplemental digital content 1, http://links.lww.com/EJGH/A544). In the multivariate analysis, the following three variables were independent risk factors associated with sarcopenia: lower BMI (OR = 0.708, 95% CI = 0.568–0.883, P = 0.002), presence of osteoporosis (OR = 3.420, 95% CI = 1.057–11.067, P = 0.040), and vertebral fracture (OR = 10.936, 95% CI = 2.678–44.663, P = 0.001) (Table S2, Supplemental digital content 1, http://links.lww.com/EJGH/A544).
These findings indicated a close relationship between osteoporosis and sarcopenia. Therefore, we assessed the correlation between osteoporosis index (BMD) and sarcopenia parameters (SMI and handgrip strength) with the Spearman’s rank correlation test (Fig. 1). There was a significant, positive correlation between SMI and BMD of the lumbar spine (r = 0.49), femoral neck (r = 0.50), and total hip (r = 0.52) (P < 0.001 for all). The handgrip strength was also significantly correlated with the BMD of the lumbar spine (r = 0.46), femoral neck (r = 0.59), and total hip (r = 0.57) (P < 0.001 for all).
Fig. 1.
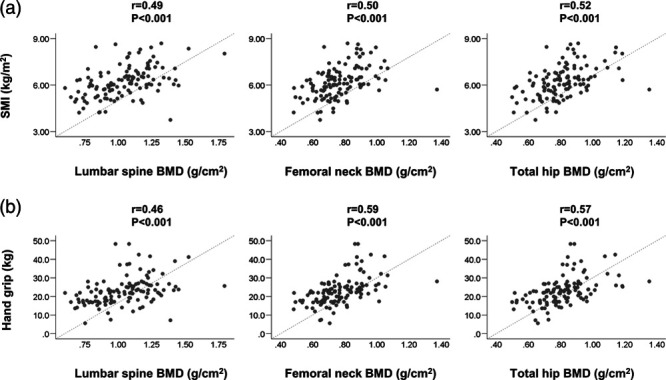
(a) Correlations between skeletal muscle mass index (SMI) and bone mineral density (BMD) of the lumbar spine, femoral neck, and total hip in patients with primary biliary cholangitis. SMI was significantly correlated with the BMD of the lumbar spine (r = 0.49, P < 0.001), femoral neck (r = 0.50, P < 0.001), and total hip (r = 0.52, P < 0.001). (b) Correlations between handgrip strength and BMD of the lumbar spine, femoral neck, and total hip in patients with primary biliary cholangitis. Handgrip strength was significantly correlated with the BMD of the lumbar spine (r = 0.46, P < 0.001), femoral neck (r = 0.59, P < 0.001), and total hip (r = 0.57, P < 0.001).
Factors associated with osteoporosis and sarcopenia in female patients
Next, we focused on the factors associated with osteoporosis and sarcopenia in female patients with PBC, given that this subpopulation is considered the most susceptible to these complications (as described in Introduction). Of the 96 female patients, 29 (30.2%) and 24 (25.0%) had osteoporosis and sarcopenia, respectively (Table S3 and S4, Supplemental digital content 1, http://links.lww.com/EJGH/A544).
The following two variables were significantly and independently associated with osteoporosis in the multivariate analysis: older age (OR = 1.074, 95% CI = 1.018–1.132, P = 0.008), and presence of sarcopenia (OR = 6.510, 95% CI = 2.100–20.180, P = 0.001) (Table S5, Supplemental digital content 1, http://links.lww.com/EJGH/A544).
The following three variables were independent risk factors associated with sarcopenia in the multivariate analysis: lower BMI (OR = 0.706, 95% CI = 0.562–0.888, P = 0.003), presence of osteoporosis (OR = 4.012, 95% CI = 1.186–13.576, P = 0.026), and vertebral fracture (OR = 8.898, 95% CI = 1.828–43.305, P = 0.007) (Table S6, Supplemental digital content 1, http://links.lww.com/EJGH/A544).
In the Spearman’s rank correlation test (Fig. S1, Supplemental digital content 1, http://links.lww.com/EJGH/A544), there was a significant, positive correlation between SMI, handgrip strength, and BMD of the lumbar spine (r = 0.45, 0.49, respectively), femoral neck (r = 0.40, 0.54, respectively), and total hip (r = 0.44, 0.54, respectively) (P < 0.001 for all).
These findings demonstrated that osteoporosis and sarcopenia are closely interrelated even in female patients with PBC. Intriguingly, sarcopenia was a significant, independent risk factor for osteoporosis and vice versa in female PBC patients, although menopause and duration of menopause were expected as leading factors associated with osteoporosis and sarcopenia (Tables S5 and S6, Supplemental digital content 1, http://links.lww.com/EJGH/A544).
Comparison of clinical characteristics between patients with and without osteoporosis/sarcopenia
We divided the 117 patients into four groups: (1) patients without both osteoporosis and sarcopenia (75/117; 64.1%); (2) patients with osteoporosis alone (15/117; 12.8%); (3) patients with sarcopenia alone (9/117; 7.7%); and (4) patients with both osteoporosis and sarcopenia (osteosarcopenia) (18/117; 15.4%) (Table S7, Supplemental digital content 1, http://links.lww.com/EJGH/A544). Patients with osteosarcopenia were significantly older and had lower levels of BMI, serum albumin and IGF-1, and higher levels of M2BPGi compared to those without both osteoporosis and sarcopenia (Fig. 2a–e). The osteosarcopenia group was significantly highest in the prevalence of vertebral fracture among the four groups (10/18; 55.6%, adjusted residual = |4.5|), whereas patients without both sarcopenia and osteoporosis had significantly lowest vertebral fracture rate (5/75; 6.7%, adjusted residual = |4.1|) (Fig. 2f).
Fig. 2.
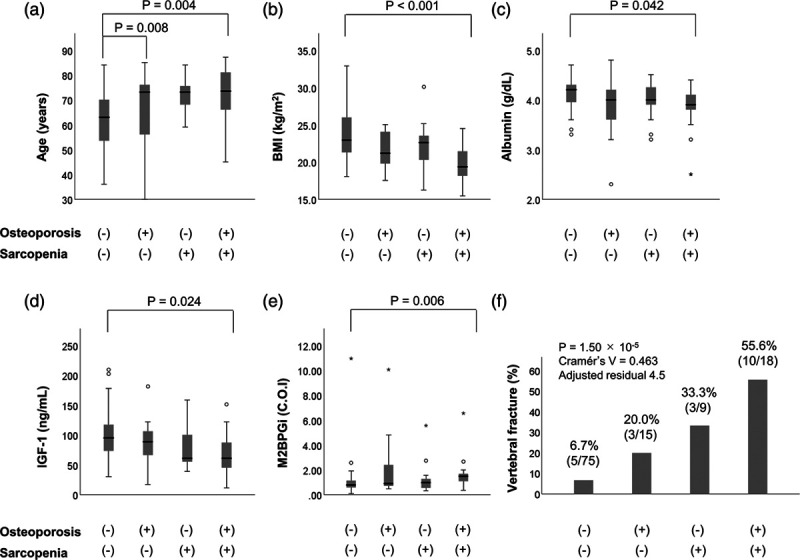
Comparison of clinical characteristics between four groups: (i) osteoporosis (−)/sarcopenia (−) group, (ii) osteoporosis (+)/sarcopenia (−) group, (iii) osteoporosis (−)/sarcopenia (+) group, and (iv) osteoporosis (+)/sarcopenia (+) (i.e. osteosarcopenia) group. The osteoporosis (+)/sarcopenia (+) (osteosarcopenia) group was (a) older, and had lower levels of (b) BMI, (c) serum albumin, and (d) IGF-1, and (e) higher levels of M2BPGi, compared to the osteoporosis (−)/sarcopenia (−) group. (f) The prevalence of vertebral fracture was significantly highest in the sarcopenia (+)/osteoporosis (+) (osteosarcopenia) group (adjusted residual = |4.5|), whereas it was significantly lowest in the osteoporosis (−)/sarcopenia (−) group (adjusted residual = |4.1|) (P = 1.50 × 10−5; Cramér’s V = 0.463). IGF-1, insulin-like growth factor 1.
Discussion
It is well known that patients with PBC are more frequently complicated by osteoporosis, compared to those with other liver diseases [3–8]. Specifically, menopausal female patients are more susceptible to osteoporosis [10,11]. Furthermore, early and late postmenopausal patients develop osteoporosis six and 10 times, respectively, more than premenopausal patients [10]. However, most notably, the present study suggested that sarcopenia was more strongly involved in osteoporosis than female and menopause. Considering that postmenopausal female account for the majority of PBC patients in a real-world clinical setting, early diagnosis and appropriate treatment of sarcopenia are becoming increasingly crucial.
To the best of our knowledge, this is the first report to demonstrate the prevalence of sarcopenia limited to PBC patients. According to the diagnosis criteria of sarcopenia proposed by the Japan Society of Hepatology [15], the prevalence was 23.1% for all patients and 25.0% for female patients in the present study, whereas it was approximately 15% for Japanese CLD (predominantly viral hepatitis) patients in previous studies [20,29]. The median age was 68 (interquartile range 57–73) years in the present study, and 65 (58–73) years [20] and 65 (54–72) years [29] in the previous studies. Additionally, the prevalence of liver cirrhosis was only 9.4% in the present study and approximately 40% in the previous studies [20,29]. These findings suggest that patients with PBC are more susceptible to sarcopenia, compared to those with other CLD. Thus, early diagnosis and appropriate treatment for sarcopenia are required especially in PBC patients, and are also beneficial for those with osteoporosis, given that sarcopenia and osteoporosis were closely interrelated with each other.
Recently, our group reported that osteoporosis is an independent predictor of sarcopenia in patients with liver cirrhosis [22]. In the present study, we found that osteoporosis was a significant, independent factor associated with sarcopenia even in PBC patients. Given that menopause is associated with a natural decline in estrogen that decreases muscle mass and strength [12], we assessed the factors associated with sarcopenia focusing on female PBC patients. Intriguingly, osteoporosis (but not menopause) was a significant, independent risk factor for sarcopenia even in female patients. Additionally, BMD levels were significantly and positively correlated with both SMI and handgrip strength values. Given these findings that there was a close correlation between the two complications, we clearly indicated that ‘osteosarcopenia’ [22–26] has now become a focus of attention in PBC. Therefore, appropriate management of osteoporosis and sarcopenia are crucial for health-related quality of life in postmenopausal female PBC patients Furthermore, we found that vertebral fracture was an independent predictor of sarcopenia in PBC patients. Vertebral fracture causes impaired physical function and immobility [30], leading to loss of muscle mass and strength. Reportedly, older women with vertebral fracture had lower muscle mass and grip strength compared to those without fracture [31,32], and sarcopenic women had 2.1 times higher risk of falls and 2.7 times higher risk of fractures than non-sarcopenic counterparts [33]. These findings indicated that there are close interrelations between sarcopenia, osteoporosis, and vertebral fracture in PBC patients.
Osteosarcopenia, defined as the coexistence of sarcopenia and osteoporosis, is a world health concern, given that it is a great risk factor for falls, fractures, disability, and mortality [22–26]. In the present study, the prevalence of osteosarcopenia was 15.4% in PBC patients. Osteosarcopenia patients were significantly older and had lower levels of BMI, serum albumin and IGF-1, and higher levels of M2BPGi than those without both osteoporosis and sarcopenia. Of note, the prevalence of vertebral fracture in the osteosarcopenia group was higher compared to those the groups without both osteoporosis and sarcopenia, and with osteoporosis or sarcopenia alone. Previous studies of older adults have shown that the osteosarcopenia group had greater impairment of physical performance and balance than the non-osteosarcopenia and sarcopenia/osteoporosis alone groups [34,35]. Consequently, osteosarcopenia conferred an increase rate of falls and fractures [34]. Another study of patients with hip fracture has reported that osteosarcopenia patients had a 1.8 times higher mortality rate than non-osteosarcopenia patients [36]. As described above, vertebral fracture results in reduced physical activity and mechanical loading on bone-muscle unit, thereby causing exacerbation of osteoporosis and sarcopenia [37]. These results suggested that osteosarcopenia is (as well as osteoporosis and sarcopenia) closely interrelated to vertebral fracture and exacerbates negative health outcomes in PBC patients.
As described above, PBC patients were occasionally complicated by osteoporosis, sarcopenia, and osteosarcopenia, which are influenced by multiple factors, including genetics, endocrine function, inflammation, and nutritional states [38]. Reportedly, vitamin D deficiency and reduced IGF-1 and gonadal sex hormones were risk factors for osteosarcopenia [37]. Indeed, lower IGF-1 levels in all patients and longer duration of menopause in female patients were significantly associated with osteoporosis and sarcopenia in our study cohort. Menopause causes a natural decline in estrogen, possibly leading to decreased bone density, and muscle mass and strength [12,13]. Estrogen deficiency increases cycle rates of bone remodeling, resorption, and formation in an unbalanced manner where resorption exceeds formation [39]. Estrogen receptors are present in muscle fibers, and the number of estrogen receptors decrease after menopause [40]. An increased number of estrogen receptors may increase muscle strength through the actions of estrogen and IGF-1 [12]. IGF-1, produced primarily in hepatocytes and some tissues including bone and muscle, regulates both bone and muscle growth through systemic and tissue-specific signaling pathways [38]. Accordingly, although sarcopenia was a significant, independent risk factor for osteoporosis and vice versa in the present study, multiple factors including decreased IGF-1 and estrogen could affect the loss of bone and muscle mass in PBC patients.
The present study had some limitations. First, histological examinations were not performed to evaluate fibrosis stage and characteristic findings. Second, life situations of patients, such as nutritional intake and physical activity, were not assessed. Third, given that the present study was a cross-sectional study, we were unable to clarify the causal relationship between osteoporosis and sarcopenia. A large-scale study including these assessments is needed to elucidate and resolve the challenges related to osteoporosis, sarcopenia, vertebral fracture, and osteosarcopenia.
Conclusion
Patients with PBC were occasionally complicated by osteoporosis, sarcopenia, vertebral fracture, and osteosarcopenia which were closely related to each other. In terms of health-related quality of life, osteoporosis and sarcopenia are critical issues, especially in postmenopausal female patients. Comprehensive diagnostic assessment and treatment strategies for these bone and muscle disorders are essential in PBC patients.
Acknowledgements
We thank the medical staff at Jikei University School of Medicine and Fuji City General Hospital who were involved in the collection of data.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.eurojgh.com.
References
- 1.Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015; 386:1565–1575. [DOI] [PubMed] [Google Scholar]
- 2.Schwinge D, Schramm C. Sex-related factors in autoimmune liver diseases. Semin Immunopathol. 2019; 41:165–175. [DOI] [PubMed] [Google Scholar]
- 3.Menon KV, Angulo P, Weston S, Dickson ER, Lindor KD. Bone disease in primary biliary cirrhosis: independent indicators and rate of progression. J Hepatol. 2001; 35:316–323. [DOI] [PubMed] [Google Scholar]
- 4.Guañabens N, Parés A, Ros I, Caballería L, Pons F, Vidal S, et al. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J Hepatol. 2005; 42:573–577. [DOI] [PubMed] [Google Scholar]
- 5.Guañabens N, Cerdá D, Monegal A, Pons F, Caballería L, Peris P, Parés A. Low bone mass and severity of cholestasis affect fracture risk in patients with primary biliary cirrhosis. Gastroenterology. 2010; 138:2348–2356. [DOI] [PubMed] [Google Scholar]
- 6.Seki A, Ikeda F, Miyatake H, Takaguchi K, Hayashi S, Osawa T, et al. Risk of secondary osteoporosis due to lobular cholestasis in non-cirrhotic primary biliary cholangitis. J Gastroenterol Hepatol. 2017; 32:1611–1616. [DOI] [PubMed] [Google Scholar]
- 7.Parés A, Guañabens N. Primary biliary cholangitis and bone disease. Best Pract Res Clin Gastroenterol. 2018; 34–35:63–70. [DOI] [PubMed] [Google Scholar]
- 8.Danford CJ, Trivedi HD, Papamichael K, Tapper EB, Bonder A. Osteoporosis in primary biliary cholangitis. World J Gastroenterol. 2018; 24:3513–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janes CH, Dickson ER, Okazaki R, Bonde S, McDonagh AF, Riggs BL. Role of hyperbilirubinemia in the impairment of osteoblast proliferation associated with cholestatic jaundice. J Clin Invest. 1995; 95:2581–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benetti A, Crosignani A, Varenna M, Giussani CS, Allocca M, Zuin M, et al. Primary biliary cirrhosis is not an additional risk factor for bone loss in women receiving regular calcium and vitamin D supplementation: a controlled longitudinal study. J Clin Gastroenterol. 2008; 42:306–311. [DOI] [PubMed] [Google Scholar]
- 11.Guañabens N, Parés A, Mariñoso L, Brancós MA, Piera C, Serrano S, et al. Factors influencing the development of metabolic bone disease in primary biliary cirrhosis. Am J Gastroenterol. 1990; 85:1356–1362. [PubMed] [Google Scholar]
- 12.Maltais ML, Desroches J, Dionne IJ. Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact. 2009; 9:186–197. [PubMed] [Google Scholar]
- 13.Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013; 9:699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. ; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016; 46:951–963. [DOI] [PubMed] [Google Scholar]
- 16.Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015; 31:193–199. [DOI] [PubMed] [Google Scholar]
- 17.Hanai T, Shiraki M, Ohnishi S, Miyazaki T, Ideta T, Kochi T, et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res. 2016; 46:743–751. [DOI] [PubMed] [Google Scholar]
- 18.Pereira FB, Leite AF, de Paula AP. Relationship between pre-sarcopenia, sarcopenia and bone mineral density in elderly men. Arch Endocrinol Metab. 2015; 59:59–65. [DOI] [PubMed] [Google Scholar]
- 19.Verschueren S, Gielen E, O’Neill TW, Pye SR, Adams JE, Ward KA, et al. Sarcppenia and its relationship with bone mineral density in middle-age and elderly European men. Osteoporos Int. 2013; 24:87–98. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi M, Abe K, Fujita M, Okai K, Takahashi A, Ohira H. Association between sarcopenia and osteoporosis in chronic liver disease. Hepatol Res. 2018; 48:893–904. [DOI] [PubMed] [Google Scholar]
- 21.Bering T, Diniz KGD, Coelho MPP, Vieira DA, Soares MMS, Kakehasi AM, et al. Association between pre-sarcopenia, sarcopenia, and bone mineral density in patients with chronic hepatitis C. J Cachexia Sarcopenia Muscle. 2018; 9:255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saeki C, Takano K, Oikawa T, Aoki Y, Kanai T, Takakura K, et al. Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet Disord. 2019; 20:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binkley N, Buehring B. Beyond FRAX: it’s time to consider “sarco-osteopenia”. J Clin Densitom. 2009; 12:413–416. [DOI] [PubMed] [Google Scholar]
- 24.Hassan EB, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician. 2017; 46:849–853. [PubMed] [Google Scholar]
- 25.Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int. 2017; 28:2781–2790. [DOI] [PubMed] [Google Scholar]
- 26.Crepaldi G, Maggi S. Sarcopenia and osteoporosis: a hazardous duet. J Endocrinol Invest. 2005; 28:66–68. [PubMed] [Google Scholar]
- 27.Working Subgroup for Clinical Practice Guidelines for Primary Biliary Cirrhosis. Guidelines for the management of primary biliary cirrhosis: the Intractable Hepatobiliary Disease Study Group supported by the Ministry of Health, Labour and Welfare of Japan. Hepatol Res. 2014; 44 (Suppl S1):71–90. [DOI] [PubMed] [Google Scholar]
- 28.WHO. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Study Group. World Health Organ Tech Rep Ser. 1944; 843:1–129. [PubMed] [Google Scholar]
- 29.Nishikawa H, Enomoto H, Yoh K, Iwata Y, Sakai Y, Kishino K, et al. Serum zinc concentration and sarcopenia: a close linkage in chronic liver diseases. J Clin Med. 2019; 8:E336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oleksik AM, Ewing S, Shen W, van Schoor NM, Lips P. Impact of incident vertebral fractures on health related quality of life (HRQOL) in postmenopausal women with prevalent vertebral fractures. Osteoporos Int. 2005; 16:861–870. [DOI] [PubMed] [Google Scholar]
- 31.Eguchi Y, Toyoguchi T, Orita S, Shimazu K, Inage K, Fujimoto K, et al. Reduced leg muscle mass and lower grip strength in women are associated with osteoporotic vertebral compression fractures. Arch Osteoporos. 2019; 14:112. [DOI] [PubMed] [Google Scholar]
- 32.Hida T, Shimokata H, Sakai Y, Ito S, Matsui Y, Takemura M, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016; 25:3424–3431. [DOI] [PubMed] [Google Scholar]
- 33.Sjöblom S, Suuronen J, Rikkonen T, Honkanen R, Kröger H, Sirola J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas. 2013; 75:175–180. [DOI] [PubMed] [Google Scholar]
- 34.Sepúlveda-Loyola W, Phu S, Bani Hassan E, Brennan-Olsen SL, Zanker J, Vogrin S, et al. The Joint occurrence of osteoporosis and sarcopenia (Osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020; 21:220–225. [DOI] [PubMed] [Google Scholar]
- 35.Drey M, Sieber CC, Bertsch T, Bauer JM, Schmidmaier R; FiAT intervention group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016; 28:895–899. [DOI] [PubMed] [Google Scholar]
- 36.Yoo JI, Kim H, Ha YC, Kwon HB, Koo KH. Osteosarcopenia in patients with hip fracture is related with high mortality. J Korean Med Sci. 2018; 33:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paintin J, Cooper C, Dennison E. Osteosarcopenia. Br J Hosp Med (Lond). 2018; 79:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawao N, Kaji H. Interactions between muscle tissues and bone metabolism. J Cell Biochem. 2015; 116:687–695. [DOI] [PubMed] [Google Scholar]
- 39.Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res. 1997; 12:498–508. [DOI] [PubMed] [Google Scholar]
- 40.Wiik A, Ekman M, Johansson O, Jansson E, Esbjörnsson M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem Cell Biol. 2009; 131:181–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


