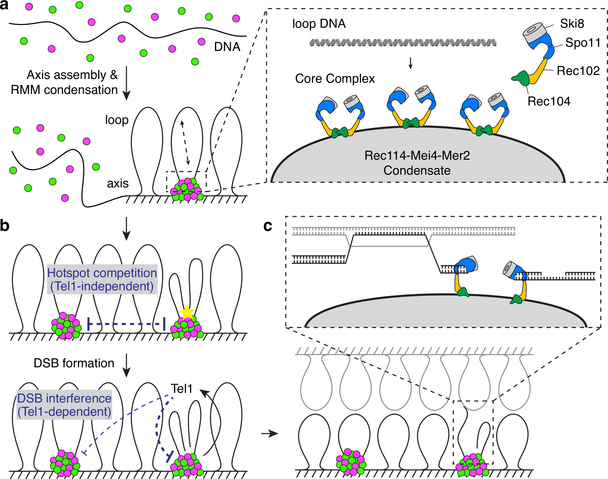
Extended Data Fig. 9: A condensate model for assembly of the meiotic DSB machinery and implications for the control of DSB formation and repair.
a. Assembly of the DSB machinery. (Left) Rec114–Mei4 and Mer2 complexes bind DNA in a highly cooperative manner to form large mixed nucleoprotein condensates. (Right) These condensates provide a platform to recruit the core complex through interactions that involve the N-terminal domain of Rec114 and the Rec102–Rec104 components of the core complex. Multiple Spo11 complexes are recruited and may engage an incoming DNA loop simultaneously. The molecular arrangement of the core complex proteins is based on ref 10. See Supplementary Discussion 4 for more detail. b. Hotspot competition and DSB interference. Competition arises prior to DSB formation as a consequence of the partitioning of RMM proteins into condensates. DSB interference is implemented through local inhibition of further DSB formation by DSB-activated Tel1. Inhibition could work on the same cluster that generated the activation DSB as well as on nearby clusters in cis. See Supplementary Discussion 5 for more detail. c. The coherence provided by the condensates may serve functions during repair, including the maintenance of a physical connection between the DNA ends that involves end-capping by condensate-embedded core complexes. See Supplementary Discussion 6 for more detail.