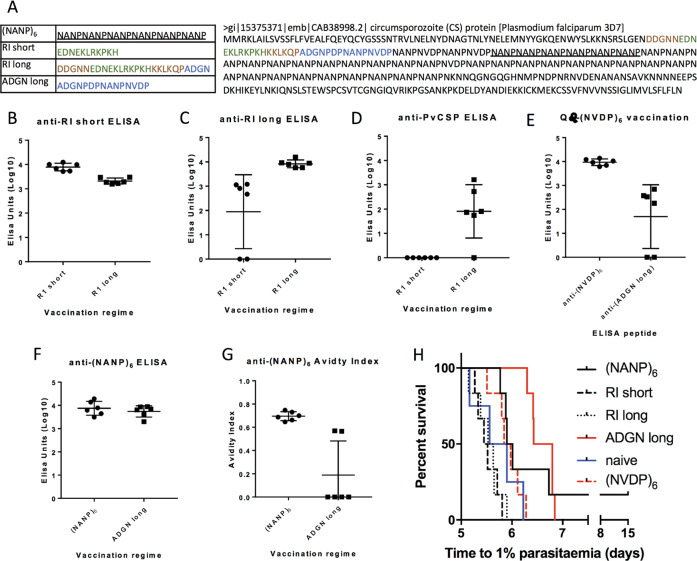
Fig. 2. Immunogenicity and protective efficacy of peptides derived from PfCSP delivered as Qβ-peptide vaccines.
BALB/c mice (n = 5–6 per group) were vaccinated with non-repeat region CSP peptides chemically coupled to Qβ (3 µg per dose, by intramuscular injection, delivered with Matrix-M™ adjuvant, three shots using 3-week intervals.) A P. falciparum CSP peptides used as Qβ-peptide vaccines and location in PfCSP sequence. Standard curve ELISAs were performed using sera taken 2 weeks after the final vaccination, against B “RI short” peptide, C “RI long” peptide, D full-length PvCSP, or F (NANP)6 peptide; E shows mice vaccinated with Qβ-(NVDP)6 and ELISAs against peptides shown on the x-axis. G Avidity index represents the ratio of sera treated with 7 M urea to untreated sera in ELISAs. Means are shown ± SD. Vaccination received shown on x-axis except in the case of (E). H Mice were challenged 3 weeks after the final shot with 1000 transgenic P. berghei sporozoites expressing PfCSP, and time to reach 1% blood-stage parasitaemia determined by linear regression using daily thin blood smears.