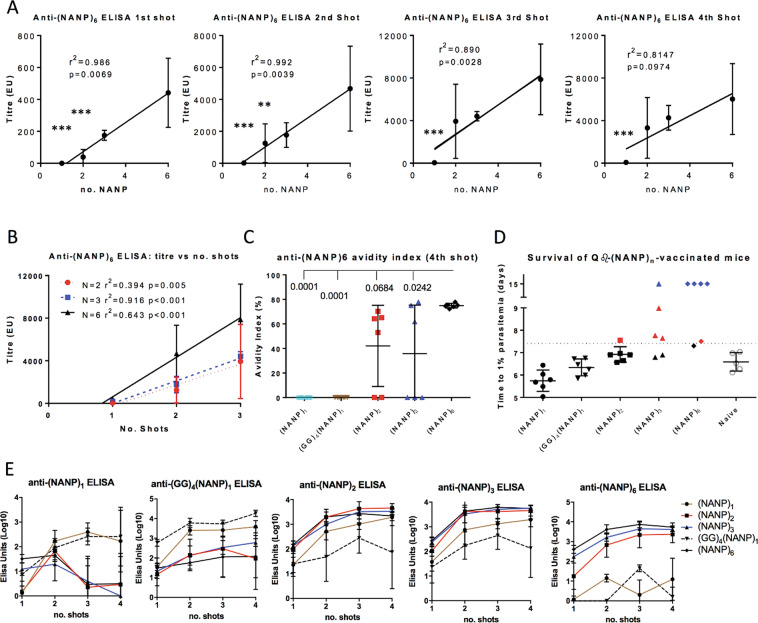
Fig. 4. Effects on immunogenicity and protective efficacy of increasing the number of units repeats in Qβ-(NANP) vaccines.
Qβ-(NANP)n VLPs were constructed by synthesising peptides with varying numbers of (NANP) unit repeats (no. NANP = 1, 2, 3, 6) and (GG)4(NANP)1 and chemically coupling them to Qβ. These VLPs were used to vaccinate BALB/c mice (n = 6 per group) by intramuscular injection of 3 µg doses in Matrix-M™ adjuvant, with four shots given at 3-week intervals. A Serum was taken 2 weeks after each shot and used in standard curve ELISAs against (NANP)6. r2 and p values from linear regressions are shown. Asterix represents significance levels of p < 0.01 (**) and p < 0.001 (***) by ANOVA in comparison to Qβ-(NANP)6. B Titre versus a number of shots for each Qb-(NANP)n group, with N = 2, N = 3 or N = 6 as shown. C The avidity indices 2 weeks post-fourth shot; shown are p values from ANOVA with Bonferroni’s multiple comparisons test in comparison to Qβ-(NANP)6-vaccinated mice. D Three weeks after the fourth vaccination mice were challenged by intravenous injection of 1000 PfCSP transgenic P. berghei sporozoites. Thin blood smears were taken daily to determine the time to reach 1% blood-stage parasitaemia by linear regression. In challenge data, points coloured blue represent sterile protection, and points coloured red to represent a delay in time-to-1%, defined as time-to-1% greater than average naïve time-to-1% plus 2 standard deviations, with the boundary represented by a dotted line. E Sera from mice in (A) were also used to determine antibody titres specific for each variable number of (NANP) repeats, 2 weeks post-vaccination; graph titles represent the peptide used to coat ELISA plates, and lines representing vaccination groups as shown. Means are shown ± SD.