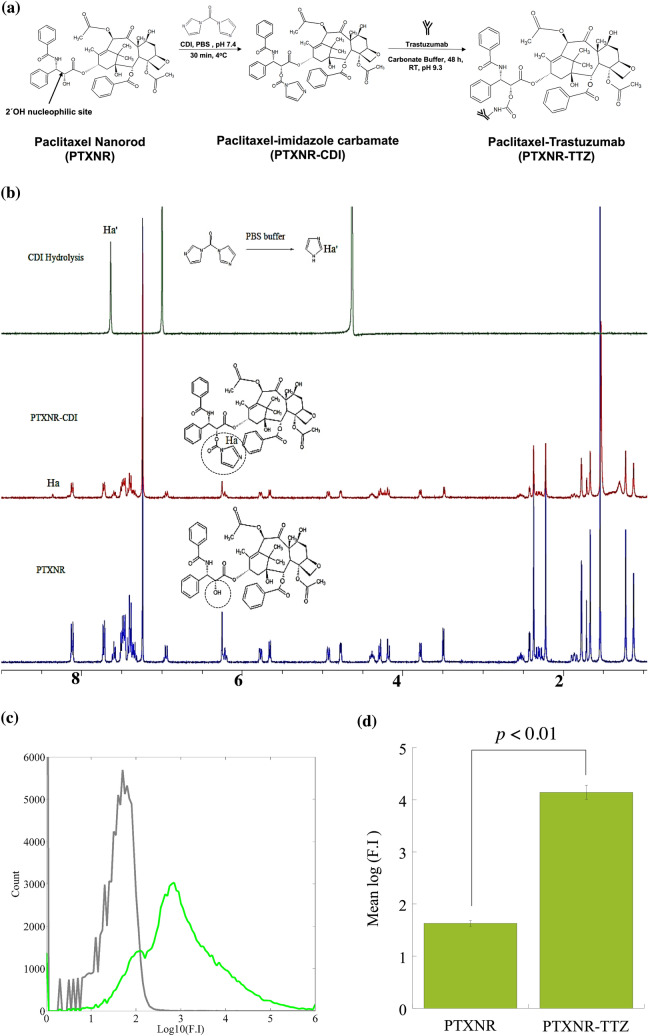
Figure 2.
1H-NMR analysis to characterize PTX-imidazole carbamate formation: (a) Schematic diagram of reactions involved for PTXNR functionalization and TTZ conjugation on the surface of PTXNRs. (b) 1H-NMR spectra of hydrolyzed carbonyldiimidazole (CDI), PTX-imidazole carbamate (PTXNR-CDI), and PTXNR. The NMR spectra were acquired at room temperature. For characterization of PTXNR and PTXNR-CDI deuterated chloroform (CDCl3, δ 7.24) and hydrolyzed CDI deuterated oxide (D2O, δ 4.65) were used as reference carrier solvents. (c) The fluorescence intensity of PTXNR-TTZ confirms the conjugation of TTZ on the surface of PTXNRs. The F.I. of bare PTXNRs (grey) and Alexa 594 fluorophore bound TTZ conjugated PTXNR (PTXNR-TTZ) (green) shows a significant increase in emission spectra. (d) The quantitative mean F.I. of PTXNR-TTZ increases by ~ 14,000 fold (2.54 logarithmic fold) compared to bare PTXNRs indicating TTZ is effectively conjugated on the surface of PTXNRs using a CDI linker.