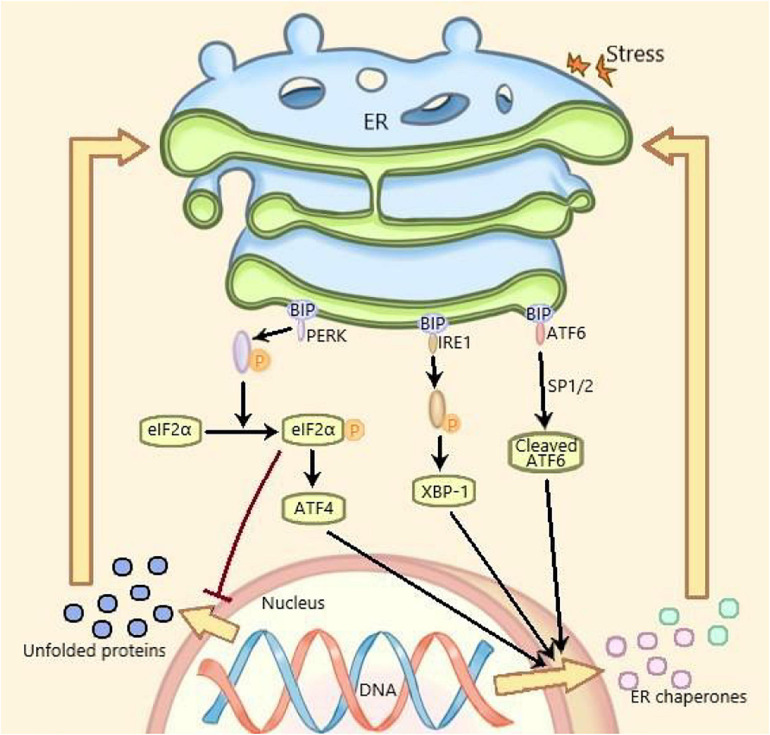
FIGURE 1.
ERS and UPR are mediated by three parallel signal transduction pathways. Binding immunoglobulin (BIP) binds to PERK, IRE1, and ATF6 to stabilize and prevent their activation under non-pressure conditions. The stressors and the unfolded proteins promote the separation of BiP from PERK, IRE1, and ATF6, thus activating these three molecules. Subsequently, the self phosphorylated PERK phosphorylated eIF2a, inhibited mRNA translation and protein synthesis, increased the expression of ATF4. The activated IRE1 cleaved XBP1 mRNA. The isolated ATF6 was cleaved by the 1-site protease (sp1) and 2-site protease (sp2) proteins of Golgi complex. Finally, the cleaved XBP1, ATF4, and spliced ATF6 promote the expression of Er chaperone genes, which are further involved in the elimination of unfolded proteins and the restoration of normal cell homeostasis. PERK, pancreatic endoplasmic reticulum kinase; IRE1, inositol-requiring enzyme 1; ATF6, activating transcription factor 6; XBP1, X-box binding protein 1; ERS, endoplasmic reticulum stress; UPR, unfolded protein response.