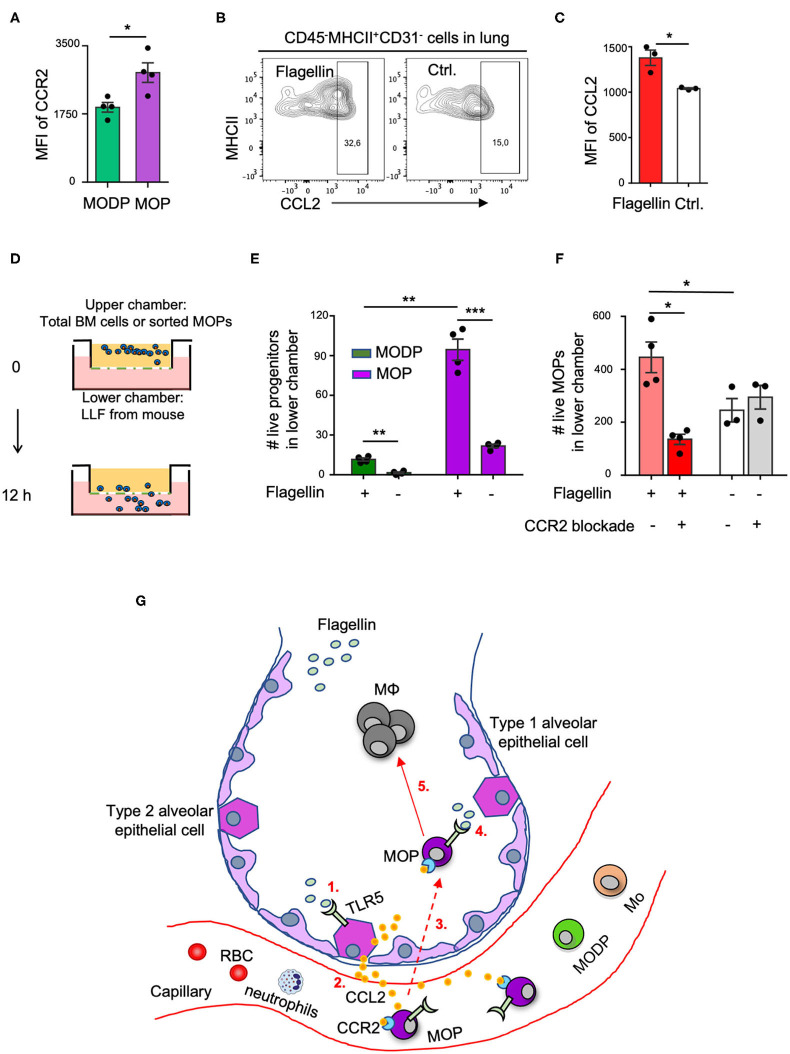
Figure 5.
The CCL2-CCR2 axis promotes Flagellin-induced MOP migration into the lung. (A) CCR2 cell surface expression on MODPs and MOPs from BM (n = 4), as analyzed by flow cytometry (MFI, median fluorescence intensity). Data are representative of two independent experiments. (B,C) CCL2 expression in type 2 AECs, defined as CD45−MHCII+CD31− cells, as determined by intracellular staining and flow cytometry in single-cell suspensions of lung tissue after overnight culture in medium with Flagellin or without (Ctrl.). (B) Representative flow cytometry plots depicting the frequencies (%) of CCL2+ cells within type 2 AECs. CCL2 FMO was used as control. (C) Representative quantitative data from one out of two independent experiments with pooled cells from 3 mice analyzed in technical triplicates. (D–F) Transwell migration assay with LLF. (D) Experimental set-up. Total BM cells (100,000 cells pooled from three mice) (E), or sorted MOPs (5,000 cells pooled from three mice) (F) were added per upper well of a 24 well or 96 well plate and allowed to migrate toward LLF from the two groups of mice (n = 3–4 each) for 12 h under the indicated conditions. Next, cells in the lower chambers were collected and absolute live cell numbers (#) of MODPs and MOPs (E) or MOPs (F) were determined by flow cytometry. Data are representative of two independent experiments. Error bars indicate SEM, and unpaired two tailed Student's t-test was used for statistical evaluation (*p < 0.05, **p < 0.01, ***p < 0.001). (G) Visual representation of the proposed model. Upon i.n. challenge with Flagellin (1), type 2 AECs secrete CCL2 (2) which attracts circulating CCR2+ MOPs into the lung (3). Subsequently, TLR5 ligation on locally recruited MOPs by Flagellin (4) promotes MΦ generation from these progenitors (5). Dashed line indicates speculative part of the model.