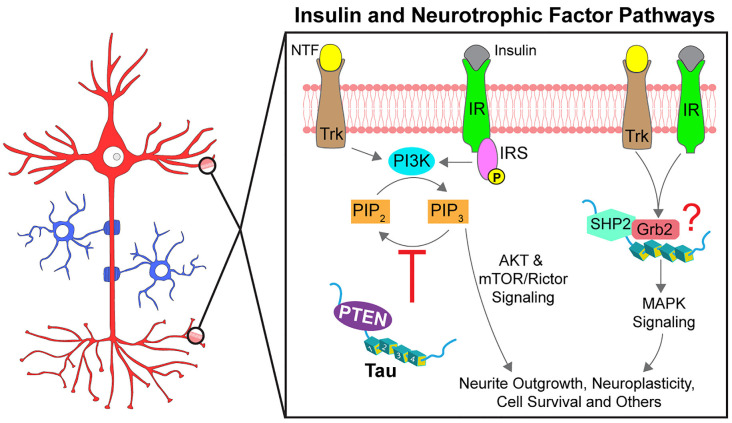
Figure 5.
Tau promotes signal transduction of insulin and neurotrophic factor pathways. Neurotrophic factors (NTF) and insulin activate specific receptor tyrosine kinases (Trks and IRs, respectively). Following autophosphorylation, the receptors initiate signaling through multiple downstream pathways, two of which are partially modulated by tau. Activation of AKT signaling by PI3K is regulated by PIP3 levels, increased through phosphorylation by PI3K and decreased through dephosphorylation by PTEN. Tau reportedly binds to PTEN directly to inhibit its phosphatase activity (red inhibitory arrow), thereby promoting AKT activation. Tau can also promote MAPK signaling through an interaction with SHP2, a tyrosine phosphatase downstream of the receptor tyrosine kinases. Tau also interacts with the SH3 domain of Grb2, an activator of SHP2 but whether tau, SHP2, and Grb2 form a complex and the downstream effects are not known (indicated by a red question mark). Phosphorylation at specific residues may impact the modulatory role of tau on these pathways.