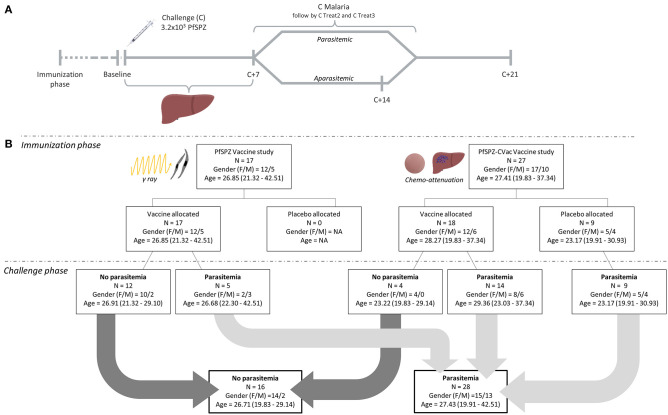
Figure 1.
Study timeline and study outcome overview. (A) The timeline represents the overview of the blood samples to investigate the kinetics of immunosuppressive cells during the controlled human malaria infection (CHMI) in the challenge phase of the malaria vaccine trials. Baseline: before the DVI of 3.2 × 103 fully infectious PfSPZ Challenge (C); C+7: 7 days after injection; C Malaria: parasitemic after fulfilling the treatment initiation end-point criteria; C Treat2 and C Treat3: subsequent days 2 and 3 of malaria treatment. C+14: 14 days after DVI for study volunteers not developing any parasitemia during CHMI; C+21: at the end of CHMI, 21 days after DVI. (B) The chart outlines detailed information on the number of participants vaccinated with either PfSPZ Vaccine or PfSPZ-CVac vaccine during the immunization phase and the respective outcome of the vaccine trials during the challenge phase. All groups are stratified by sex, age, vaccine and study arm. Age is reported in years as median and range; and sex as female/male (F/M) ratio.