Abstract
The islets of Langerhans constitute the endocrine pancreas which regulates blood glucose homeostasis and their dysfunction results in diabetes. Each of the pancreatic islets constitutes an entire micro-organ with intricate cell to cell interactions and that is well vascularized and innervated. An important therapeutic advantage in islet transplant is that pancreatic islets maintain their organ integrity when isolated and transplanted to patients with severe diabetes. Once transplanted, the islet micro-organs actively contribute to their own vascularization and start to function immediately. Hence, in terms of organ transplantation, the application of pancreatic islets will be a decisive clinical tool for future diabetes care (credit: Tilo Moede).
Keywords: Diabetes, Islet transplant, Islet transplantation, Isolated islets, Micro-organ, Pancreatic islets
The endocrine part of the pancreas, the islets of Langerhans, constitutes 2% of the pancreatic volume. Although we have more than one million islets in the human pancreas, each one of them constitutes an entire organ that is well vascularized and innervated1–4. This micro-organ is 50–500 micrometers in diameter and contains 2,000–5,000 cells that are mainly the insulin-secreting beta cells, the glucagon-secreting alpha cells, and the somatostatin-secreting delta cells (Figure 1). Not only are these cells regulated by a sophisticated inter-play between nutrients in the blood and neurotransmitters released from nerves, but also by intrinsic paracrine signals5,6. Therefore, for islet cells to have their proper physiological function in regulating blood glucose homeostasis, they need to be within the structure of the micro-organ. If islet cells are dissociated, their function is impaired. Hence, the ultimate importance of these micro-organs is well accepted, and their failure will undoubtedly lead to diabetes development. In this context, a big emphasis is put on defects in the insulin-secreting beta cells, but also dysfunctional alpha and delta cells are likely to play a role in diabetes development. An illustration of the fundamental importance of the pancreatic islet micro-organ in regulating glucose homeostasis is the observation that it serves as the systemic “glucostat” and that determines the glycemic set point7.
Figure 1.
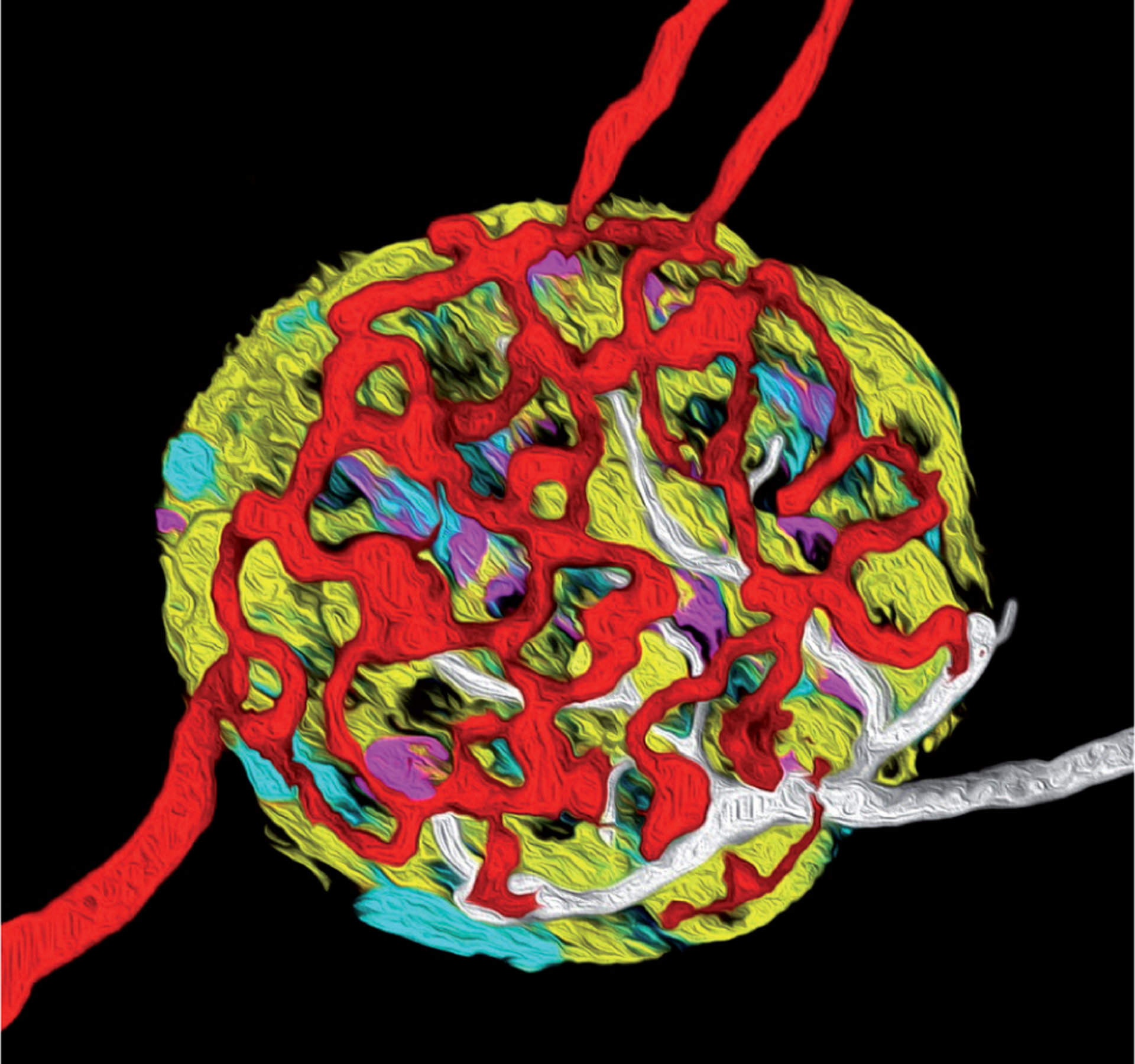
Artistic interpretation of a confocal 3D reconstruction of a pancreatic islet highlighting the complexity of this micro-organ with intricate interactions among the endocrine cells, blood vessels, and nerves. Depicted in yellow are beta cells, in cyan alpha cells, in magenta delta cells, in red blood vessels, and in white nerves. The depiction is based on a prototypical rodent islet with a core of beta cells (accounting for up to 90% of the islet endocrine cells) and with alpha and delta cells in the mantle.
The fact that the islets of Langerhans constitute entire micro-organs has been taken advantage of not only in basic research but also in the clinic with regard to islet transplantation. In basic research, it is important to understand endocrine cell to cell interactions as well as endocrine cell interactions with blood vessel endothelial cells and nerves. These interactions form the basis for activation of distinct signaling networks that will then lead to the activation of respective cells. Information obtained from such micro-organ studies in the living organism is fundamental to our abilities to identify novel druggable targets and drugs that interact with these targets for treatment of diabetes. In transplantation of pancreatic islets to patients with type 1 diabetes, the relative ease by which the islets can be isolated and injected into the recipient is an important consideration when embarking on islet transplantation as a clinical procedure to treat diabetes. Since the pancreatic islet constitutes an entire micro-organ, it will actively contribute to its own engraftment (e.g., vascularization) and start to function after transplantation to its new site. These transplanted islets have the capacity to replace the endogenous endocrine pancreas and thereby regulate glucose homeostasis.
Although there is a lot of promise in islet transplantation as a clinical procedure to cure diabetes, there is still a number of problems that need be solved prior to this procedure reaching its full therapeutic potential. Many of these problems relate to the immunological reactions both in terms of allogeneic rejection and recurrence of autoimmunity, lack of high-quality islet micro-organs for transplantation and inappropriate locations for islet transplantation.
Moving forward, it should be emphasized that each islet on its own constitutes a well-functioning entire organ and should be viewed as such when considering islet transplantation as a routine treatment strategy to help patients with severe diabetes. Hence, there is no reason that islet transplantation should be governed by rules and regulations other than organ transplantation in general.
Acknowledgements:
The authors would like to acknowledge Tilo Moede for help with the schematic of the islet. They also acknowledge colleagues on prior works that supported the opinions expressed in this work.
Funding:
This work was supported in part through funds from the Diabetes Research Institute Foundation (DRIF) and the Diabetes Wellness Foundation; and through grants from the National Institute of Allergy and Infectious Diseases (NIAID) – R56AI130330 – the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) – F32DK083226 and K01DK097194. It was also supported in part by the Swedish Research Council, the Family Erling-Persson Foundation, the Novo Nordisk Foundation, the Stichting af Jochnick Foundation, the Swedish Diabetes Association, the Berth von Kantzow’s Foundation, the Strategic Research Program in Diabetes at Karolinska Institutet, the European Research Council grant ERC-2018-AdG834860EYELETS, the Swedish Foundation for Strategic Research, and the Knut and Alice Wallenberg Foundation.
Footnotes
Conflict of Interest:
The authors declare that they have no conflict of interest to disclose in relation to this work. The funders were not involved in this work and the views expressed in it are solely those of the authors.
References
- 1.Abdulreda MH, Rodriguez-Diaz R, Cabrera O, Caicedo A, Berggren PO. The Different Faces of the Pancreatic Islet. Adv Exp Med Biol 2016; 938: 11–24. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Diaz R, Speier S, Molano RD, Formoso A, Gans I, Abdulreda MH, Cabrera O, Molina J, Fachado A, Ricordi C, Leibiger I, Pileggi A, Berggren PO, Caicedo A. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proc Natl Acad Sci U S A 2012; 109: 21456–21461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyqvist D, Speier S, Rodriguez-Diaz R, Molano RD, Lipovsek S, Rupnik M, Dicker A, Ilegems E, Zahr-Akrawi E, Molina J, Lopez-Cabeza M, Villate S, Abdulreda MH, Ricordi C, Caicedo A, Pileggi A, Berggren PO. Donor islet endothelial cells in pancreatic islet revascularization. Diabetes 2011; 60: 2571–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 2011; 14: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren PO, Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med 2011; 17: 888–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molina J, Rodriguez-Diaz R, Fachado A, Jacques-Silva MC, Berggren PO, Caicedo A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes 2014; 63: 2714–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Diaz R, Molano RD, Weitz JR, Abdulreda MH, Berman DM, Leibiger B, Leibiger IB, Kenyon NS, Ricordi C, Pileggi A, Caicedo A, Berggren PO. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab 2018; 27: 549–558.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]


