Scheme 3. Couplings of a Site-Selectively Functionalized Lycodine Congener in the Syntheses of C2-Substituted Alkaloids.
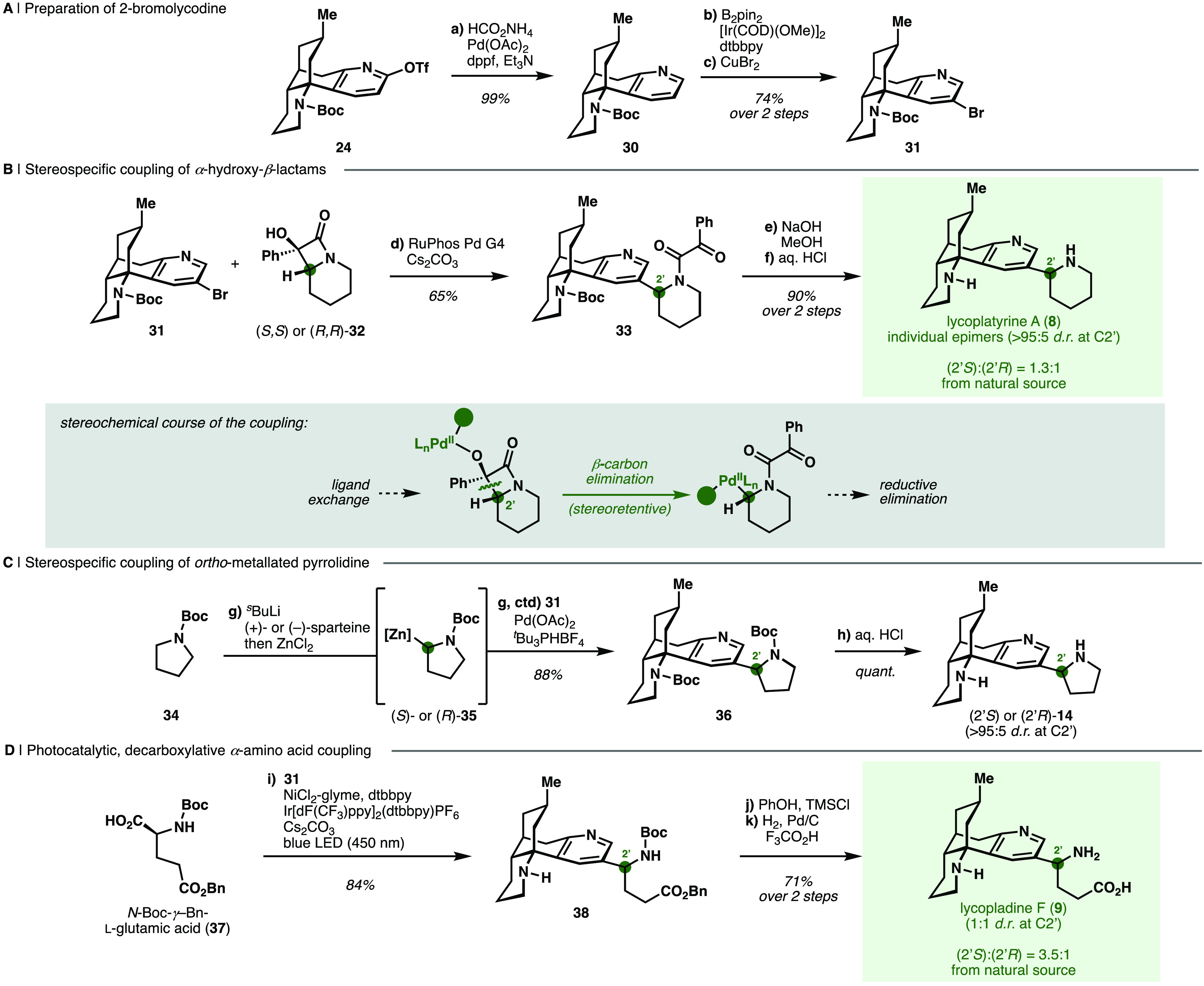
Reagents and Conditions: (a) HCO2NH4, Pd(OAc)2, dppf, Et3N, DMF, 60 °C (99%); (b) B2 pin2, [Ir(COD)(OMe)]2, dtbbpy, THF, 80 °C; (c) CuBr2, MeOH, H2O, 80 °C (74%, 2 steps); (d) RuPhos Pd G4, Cs2CO3, toluene, 70 °C [(2′S)-33: 65%, (2′R)-33: 65%, 33 as epimeric mixture at C2′ with rac-32: 72%]; (e) NaOH, MeOH, 1,4-dioxane, 70 °C (f) aq. 6 M HCl, 70 °C [(2′S)-8: 90%, (2′R)-8: 68%; 2 steps]; (g) sBuLi, (+)- or (−)-sparteine, MTBE, −78 °C, then ZnCl2, THF, −78 to 21 °C, then 31, Pd(OAc)2, tBu3PHBF4, MTBE, 60 °C [(2′S)-36: 55%, (2′R)-36: 88%]; (h) aq. 6 M HCl, 21 °C [(2′S)-14: quantitative, (2′R)-14: 70%]; (i) 31, NiCl2-glyme, dtbbpy, Ir[dF(CF3)ppy]2(dtbbpy)PF6, Cs2CO3, DMF, 450 nm LED, 21 °C (84%); (j) PhOH, TMSCl, CH2Cl2, 21 °C; (k) 500 psi H2, Pd/C, CF3CO2H, MeOH, 21 °C (71%, 2 steps).
