Fig. 3. Metamorphic folding is enabled by the combined presence of changes in dimer interface, structural flexibility, and residue contact networks.
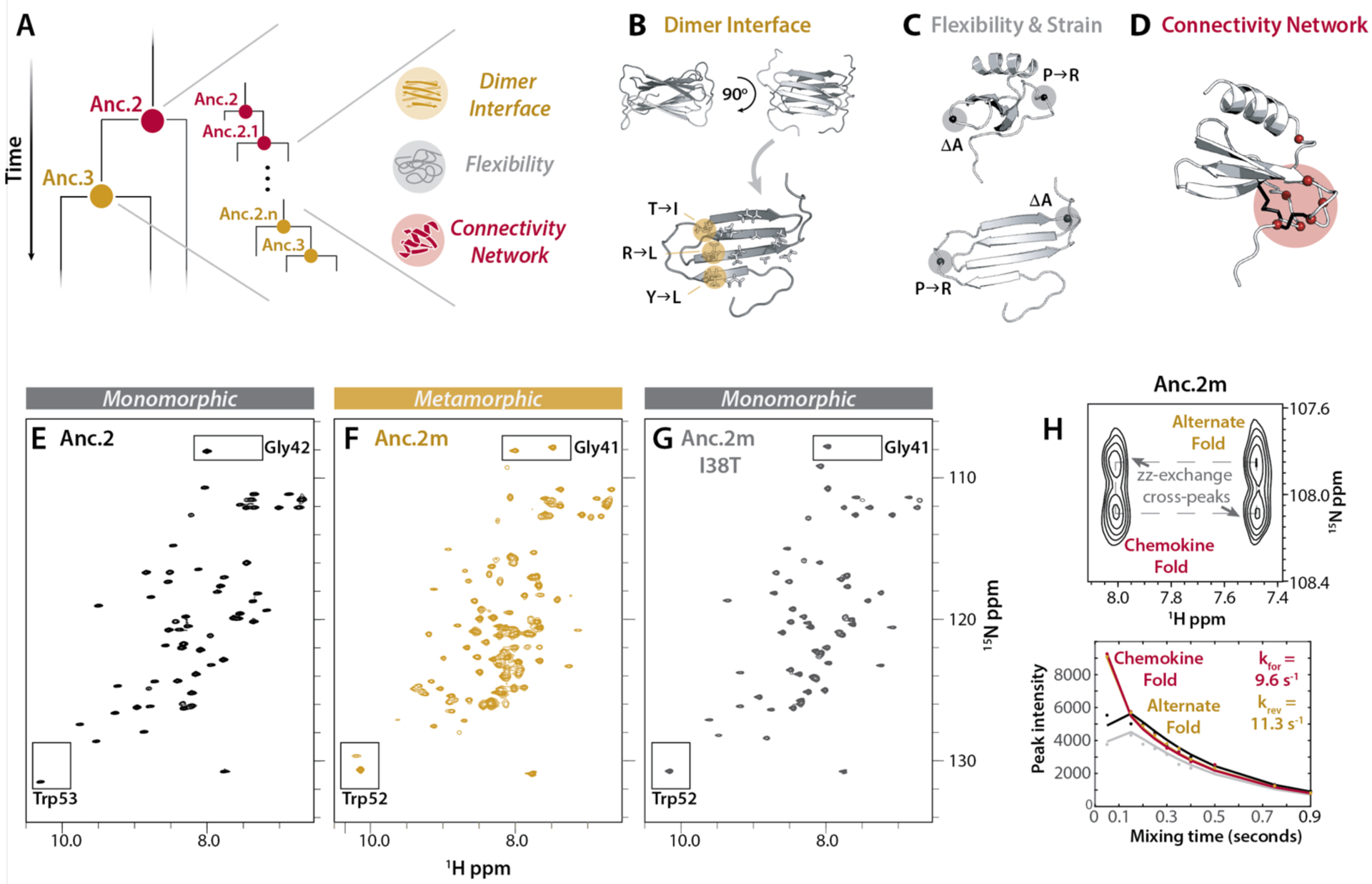
(A) Schematic of the hypothesis that metamorphosis evolved over time via a subset of key sequence changes from Anc.2 towards Anc.3. (B) Dimer interface positions that are polar in Anc.2 and hydrophobic in Anc.3. (C) Positions likely to be important for structural flexibility, and creation of strain in the chemokine fold. (D) Positions selected for replacement in Anc.2 using contact network analysis (Cα, red spheres). Anc.0 disulfides shown in black. (E-G) HSQC spectra for Anc.2 (E), Anc.2m (F) and Anc.2m I38T (G) (50°C, 20 mM NaPO4 (pH 6.0)). Boxes: conformational reporter residues. (H) ZZ-exchange analysis for Anc.2m (50°C, 20 mM NaPO4 (pH 6.0)) using reporter residue Gly41. Top: cross peaks indicate interconversion. Bottom: curves fit to peak intensities vs. time to calculate kinetic parameters. kforward (kfor), interconversion from the alternate fold towards the chemokine fold; kreverse (krev), interconversion in the reverse direction.
