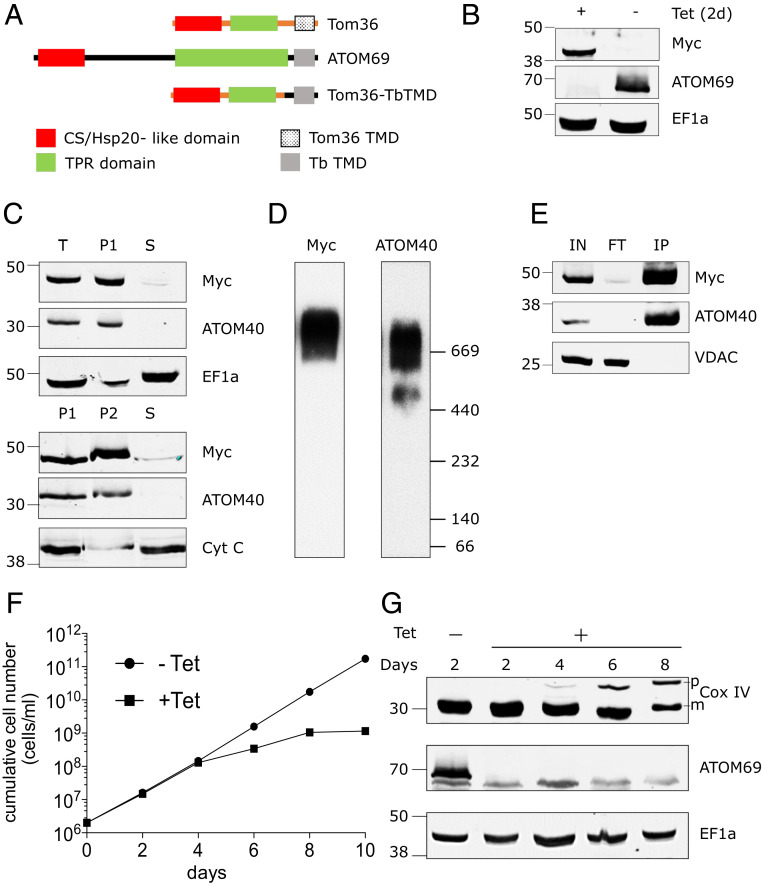
Fig. 4.
Chimeric Trichomonas Tom36 import receptor integrates into the ATOM complex. (A) Domain structure of T. vaginalis Tom36, ATOM69, and its chimera (Tom36-TbTMD). Tom36-TbTMD is expressed in the background of ATOM69 3′-UTR RNAi. (B) Immunoblots probed with antisera against c-Myc (Myc) and ATOM69 show the efficient replacement of endogenous ATOM69 by the chimeric Tom36-TbTMD. Elongation factor 1a (EF1a) serves as a loading control. (C) Immunoblot analysis of total-cell (“T”), digitonin-extracted mitochondria-enriched pellet (P1), and soluble (“S”) fractions of cells expressing c-Myc–tagged chimeric protein (Top). ATOM40 and EF1a serve as mitochondrial and cytosolic markers, respectively. The digitonin-extracted mitochondria-enriched pellet (P1) was subjected to carbonate extraction. Immunoblot analysis of total (P1), pellet (P2), and soluble fraction (“S”) after carbonate extraction (Bottom). ATOM40 and cytochrome C (Cyt C) serve as integral and peripheral membrane markers, respectively. (D) Crude mitochondrial fractions of the tagged Tom36-TbTMD–expressing cell line were analyzed by BN-PAGE. The corresponding immunoblots were probed using antisera against c-Myc (Myc) and ATOM40. (E) Coimmunoprecipitation using tagged Tom36-TbTMD (Myc) as the bait. Voltage-dependent anion channel (VDAC) serves as a negative control. (F) Growth curves of uninduced (−Tet) and induced (+Tet) ATOM69 3′-UTR RNAi cell line ectopically expressing the tagged Tom36-TbTMD. (G) Immunoblots showing steady-state levels of CoxIV and ATOM69 in whole-cell extracts of the same cell line as in F collected at the indicated time points after Tet induction. EF1a serves as loading control.