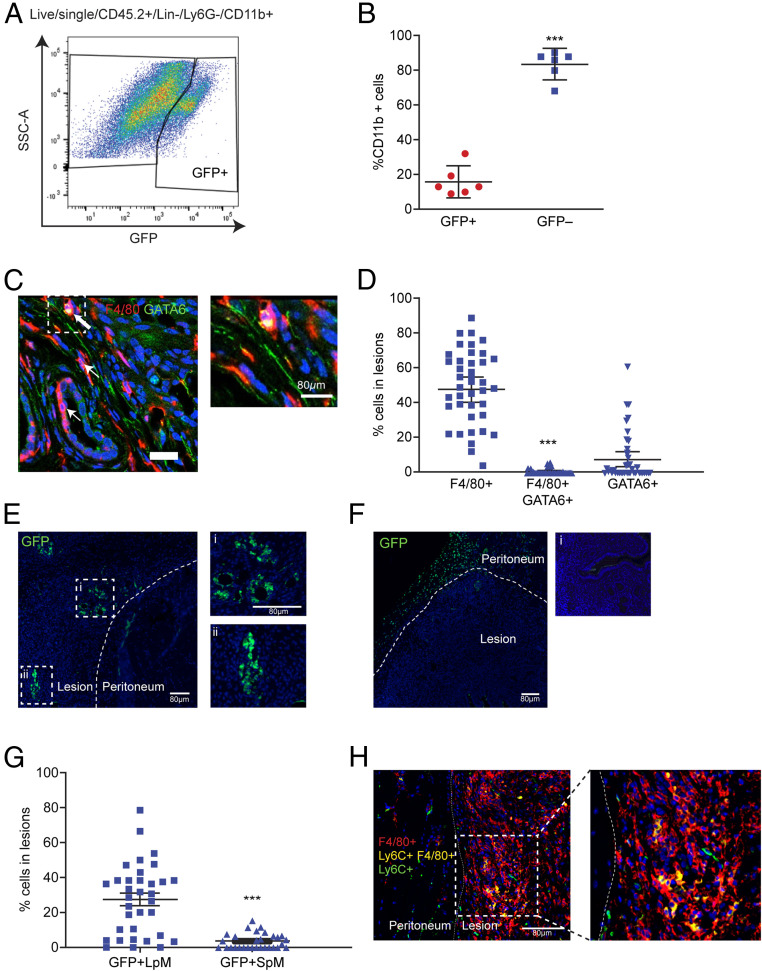
Fig. 1.
Lesion-resident macrophages have different origins. Donor endometrial tissue from MacGreen mice was injected into the peritoneal cavity of wild-type recipient mice to assess incorporation of endometrial macrophages into lesions. Lesions were collected at 2 wk after tissue injection in each of the separate studies presented in this figure. (A) Expression of GFP by lesion-resident macrophages recovered from MacGreen (donor) to wild-type (recipient) endometrial transfers (n = 6). (B) Quantification of donor endometrial-derived (GFP+) macrophages vs. recipient-derived (GFP−) macrophages. Dual immunodetection for identification of LpM in lesions. (C) Dual immunodetection for F4/80 (red) and GATA6 (green; n = 7 mice [10 lesions]). Thick arrows indicate dual-positive cells and thin arrows indicate GATA6− macrophages. (D) Quantification of F4/80+, dual-positive and GATA6+ cells in lesions. Fewer than 1% of cells were dual-positive for F4/80 and GATA6. Adoptive transfers of MacGreen peritoneal macrophages into wild-type mice with GFP immunodetection to assess incorporation of LpM and SpM into lesions. (E and F) Immunofluorescence for GFP on lesions collected following adoptive transfer of approximately 1 × 106 LpM (E) or SpM (F) isolated from MacGreen mice. Curved dotted line indicates the boundary between peritoneal and lesion tissue. In E, i and ii show magnified images; in F, i shows a negative control. (G) Quantification of GFP+ LpM and SpM in lesions. Dual immunodetection for Ly6C+ monocytes in lesions. (H) Dual immunodetection for F4/80 (red) and Ly6C (green) performed on mouse lesions. Data are presented as mean with 95% confidence intervals. Statistical significance was determined using a Student’s t test. ***P < 0.001.