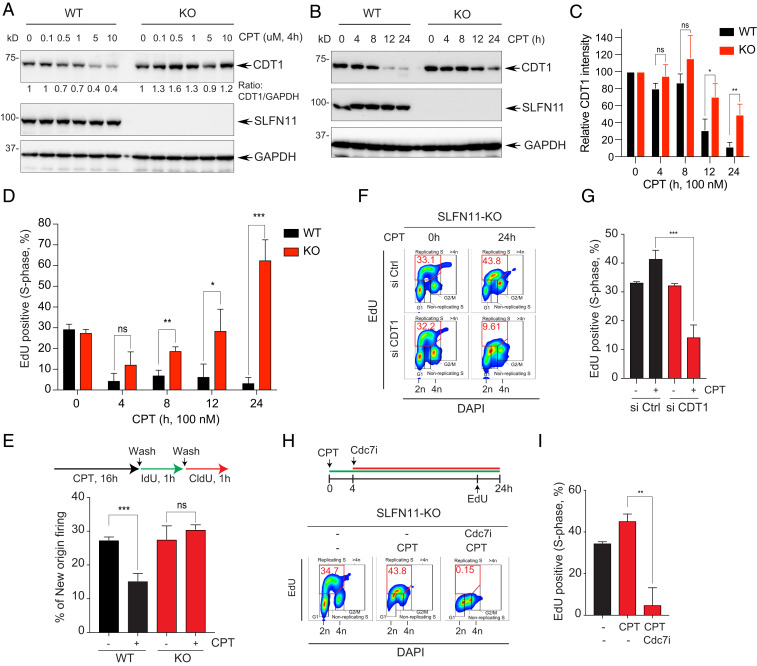
Fig. 4.
Defective CDT1 degradation causes replication recovery in SLFN11-deficient cells. (A) DU145 SLFN11-WT and -KO cells were treated with the indicated concentrations of CPT for 4 h. Protein levels were analyzed by Western blotting. (B) DU145 SLFN11-WT and -KO cells were treated with CPT (100 nM) for the indicated times. Levels of protein expression were analyzed by Western blotting. (C) Quantitation of CDT1 as shown in B. Bars show the mean band intensity from triplicate experiments, normalized to GAPDH. *P < 0.0274, **P < 0.0077. (D) Percentage of EdU+ S-phase cells in the time course treatment of CPT was determined by flow cytometry. Error bars represent SD (n = 3). *P < 0.0335, **P < 0.0025, ***P < 0.0005 Student’s t test. (E, Upper) Labeling protocols for the DNA combing assay. Cells were treated with CPT (100 nM) for 16 h and then pulse-labeled sequentially with IdU (100 µM) and CldU (100 µM) for 1 h. (Lower) Frequencies of new origins only labeled by the CldU pulse. Error bars represent SD (n = 3). ***P < 0.0009 Student’s t test. (F) CDT1-dependent replication recovery. SLFN11-KO cells were transfected with siControl (Ctrl) or siCDT1 for 48 h, and then treated with CPT for 24 h. Cells were incubated with EdU (10 µM) for 30 min prior to harvest. The percentage of EdU+ and DAPI+ cells was determined by flow cytometry. (G) Quantification of the EdU+ cells in S-phase. Error bars represent SD (n = 3). ***P < 0.0009 Student’s t test. (H, Upper) Treatment protocol. Cells were treated with CPT for 24 h and with the Cdc7 inhibitor (PHA-767491, 5 µM) after 4 h of CPT treatment. Cells were incubated with EdU (10 µM) for 30 min prior to harvest. Percentage of EdU+ and DAPI+ cells was determined by flow cytometry. (I) Quantification of the EdU+ cells in S-phase. Error bars represent SD (n = 3). **P < 0.008 Student’s t test.