Abstract
The COVID-19 pandemic has the potential to affect the human microbiome in infected and uninfected individuals, having a substantial impact on human health over the long term. This pandemic intersects with a decades-long decline in microbial diversity and ancestral microbes due to hygiene, antibiotics, and urban living (the hygiene hypothesis). High-risk groups succumbing to COVID-19 include those with preexisting conditions, such as diabetes and obesity, which are also associated with microbiome abnormalities. Current pandemic control measures and practices will have broad, uneven, and potentially long-term effects for the human microbiome across the planet, given the implementation of physical separation, extensive hygiene, travel barriers, and other measures that influence overall microbial loss and inability for reinoculation. Although much remains uncertain or unknown about the virus and its consequences, implementing pandemic control practices could significantly affect the microbiome. In this Perspective, we explore many facets of COVID-19−induced societal changes and their possible effects on the microbiome, and discuss current and future challenges regarding the interplay between this pandemic and the microbiome. Recent recognition of the microbiome’s influence on human health makes it critical to consider both how the microbiome, shaped by biosocial processes, affects susceptibility to the coronavirus and, conversely, how COVID-19 disease and prevention measures may affect the microbiome. This knowledge may prove key in prevention and treatment, and long-term biological and social outcomes of this pandemic.
Keywords: COVID-19, microbiome, hygiene hypothesis
Humans are at a major crossroads of two major biosocial processes affecting the microbes that collectively inhabit us (our microbiome). The first process is the continued loss of gut microbial diversity and ancestral microbes among a large swath of the world’s population. This loss of diversity has accelerated over the past several decades, likely affecting the coexistence between humans and our microbial residents and human health through the development of noncommunicable diseases, including obesity, asthma, cardiovascular diseases, and brain diseases (1). The second process, the COVID-19 pandemic, is occurring at a breakneck pace across the planet, with diverse consequences for its populations. Large-scale pandemics entail widespread pathogen transfer between individuals and disruption of human activity, and they presumably affect microbial diversity and richness in infected and uninfected individuals. The interaction of these two processes is of critical importance for the collective human microbiome and, more broadly, for human health.
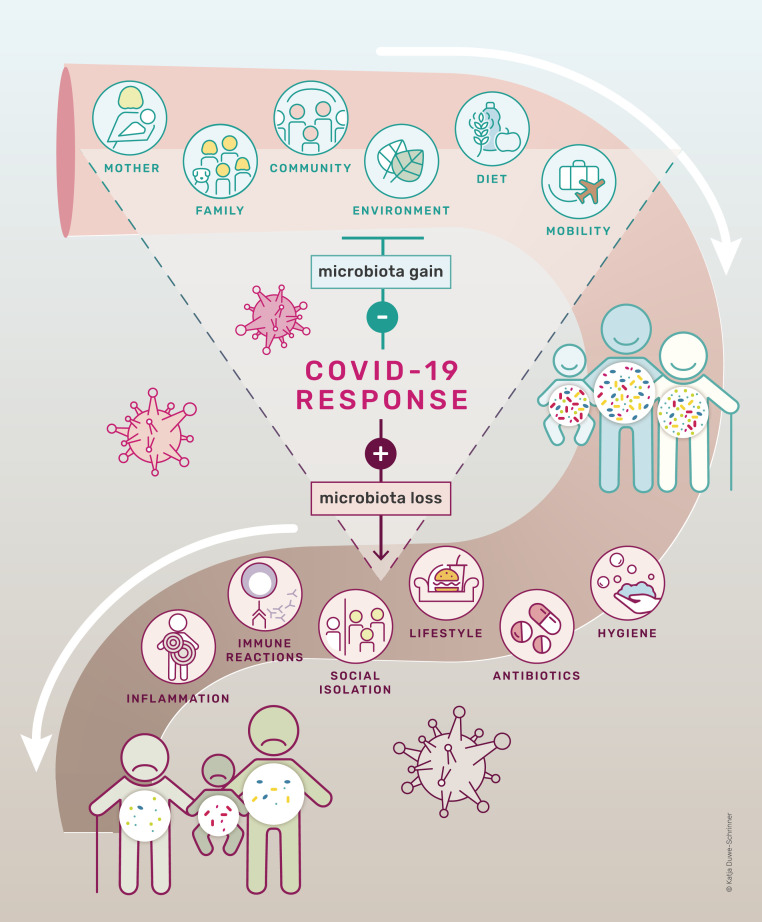
The model in Fig. 1 outlines the process by which microbial diversity is lost. Gut microbial richness results from a balance of the acquisition and the loss of microbial species. The original hygiene hypothesis, first framed by David Strachan (2), has evolved into new, more complex and explicit hypotheses that capture many of the processes that influence gut microbial establishment and extinction (3, 4). The most recent versions maintain that multiple changes among some of the world’s populations have occasioned a loss of microbial diversity, which has accelerated over the last century because of many processes and practices: increased urbanization; overuse of antibiotics and other medications; birth and infant feeding practices; intensified hygienic practices that disinfect bodies, homes, and workplaces; reduced diversity in global diets (especially declining intake of dietary fiber and increased consumption of processed foods); and widespread use of tobacco, alcohol, and other drugs (5–7).
Fig. 1.
A proposed model of how COVID-19 measures influence microbiota diversity during the lifetime of an individual. While some environmental factors foster microbial diversity, others, such as intensive hygiene and antibiotics, negatively affect microbial diversity. COVID-19 measures prevent acquisition of microbiota diversity and accelerate microbiota loss.
Reduced acquisition and increased depletion of microbes over generations may lead to the extinction of microbial species ancestrally associated with humans; species may be permanently lost from the microbial pool unless reinoculation from other sources occurs. First proposed by Blaser and Falkow (8) and independently by Rook (9), this longer-term loss is known as the disappearing microbiota hypothesis. Reduced microbial exposure resulting from diverse social changes and associated increases in host inflammation have been linked to rising rates of chronic diseases, including obesity, diabetes, asthma, and various autoimmune diseases (10). Disruption of the microbiome predisposes us to multiple seemingly nontransmissible human diseases. Germ-free animals, devoid of a microbiome, develop a high titer of IgE, the antibody isotype associated with allergic inflammation (11); loss of immune cells reacting to bacteria leads to severe allergic inflammation (12). In humans, exposure to rural environments and farm animals decreases the risk of allergy (13), whereas antibiotic consumption during early age is associated with an increased risk of developing allergic, autoimmune, and metabolic disease (4), presumably by affecting the balance of acquisition and loss of microbes. Notably, they may also heighten our susceptibility to infectious disease, just as climate change, deforestation, factory farms, and global connectivity intensify the likelihood of novel infectious disease pandemics (14).
This process of microbial diversity loss is occurring unevenly across the planet. Clean water, soap, and sanitation are not equally distributed to all people; access to and use of antibiotics, however, are widespread in low- and middle-income countries (LMICs), constituting a “quick fix infrastructure,” even for the poorest populations (15, 16). Moreover, multiple vulnerable populations—urban residents, racial and ethnic minorities, migrants, low-income earners (17)—disproportionately suffer from certain chronic diseases linked to altered microbial functionality.
The COVID-19 pandemic itself is, of course, nested in a much longer history of pandemics that have afflicted humankind. From the Neolithic agricultural revolution, when larger human settlements facilitated the circulation of pathogens between humans and their domesticated animals, human history is punctuated with the repeated suffering of large epidemics and their disruptive consequences (18). Although notions of infection and practices of hygiene have varied across space and time, people have long used certain practices to manage pandemics: fleeing endemic areas, physical distancing, separating the sick from the healthy, and scapegoating of certain groups, often the most vulnerable to falling ill and dying. From the Black Death in the 14th century through smallpox epidemics in the 18th century, cholera in the 19th century, and the influenza pandemic of 1918–1919, pandemics have weighed most heavily on the poor, migrants, and ethnic and racial minorities (19). As with past pandemics, COVID-19 mirrors and exacerbates existing inequalities, so that aging, poor, and chronically ill populations suffer much higher morbidity and mortality (20).
The collision of the current pandemic with our decades-long process of hygienic and accompanying microbial changes, and the recent recognition of the importance of establishing and maintaining a healthy microbiome, provides a unique opportunity to explore, in real time, several key questions about humans and their microbiomes (Table 1). This moment can provoke investigation of how social inequalities, the human microbiome, and risk factors such as age or chronic disease affect susceptibility to the most serious outcomes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. It also allows examination of how COVID-19 control measures interact with various human practices and socioeconomic and ecological conditions that determine and modify microbial composition, the stability of these interrelations, and their capacity to establish or reestablish healthy microbial composition.
Table 1.
Societal practices affected by COVID-19 response measures that impact the microbiome
| Practice | Pre-COVID | COVID response measures | Consequence (and most affected populations) | Further research and possible recommendations |
| Hygiene | Some wealthy populations: loosening hygienic practices | Intensive disinfection and hygiene in wealthy countries | Loss of microbial diversity | How can we increase healthy microbial exposure? (outdoors, diet, etc.) |
| LMICs: access to clean water, soap, sewage disposal inaccessible or very uneven | Some in LMICs, but clean water, soap, sewage disposal remains inaccessible or very uneven | May interact with food shortages and antibiotics to lead to loss of microbial diversity | Increase access to soap, clean water, masks; long-term investment in reliable sewage systems | |
| Food | Uneven, with existence of “double burden” (malnutrition and obesity) | Mixed in wealthy countries: some healthier eating, but also rising risk of obesity from high processed food consumption, inactivity | Loss of microbial diversity | Healthier, balanced food assistance (increased fiber); probiotics? |
| Uneven in LMICs, with significant malnutrition, including “double burden” (malnutrition and obesity) | LMICs: food production disrupted, with rising malnutrition | Loss of microbial diversity (poor, vulnerable populations) | Healthier, balanced and more widespread food assistance | |
| Antimicrobial use (including antibiotics) | High | High | Loss of microbial diversity | Discourage antimicrobial use when not needed |
| Social interaction and mobility | Intensive | Mixed | Loss of microbial diversity, particularly among elderly, very young | Permit interactions within social bubbles; allow outdoor access in urban areas |
In this Perspective, we explore, first, what we know about the human microbiome’s influence on COVID-19, and then examine in greater depth the current pandemic’s potential effects on the human microbiome. We draw lessons from these intersecting processes and identify critical questions that should be tackled simultaneously by biomedical and social sciences researchers and public health actors for our near- and longer-term futures.
COVID-19 and the Microbiome
At this writing, we have little direct evidence of interactions between the human microbiome and SARS-CoV-2 infection (21). We do not know how the composition or metabolic activity of microbial populations living on mucosal surfaces (airway epithelial cells, gastrointestinal enterocytes) of the human body affect initial susceptibility to SARS-CoV-2 infection, subsequent pathogenesis, or outcome. Some intriguing observations, however, make this possibility difficult to ignore. Framing these unknown biological interactions are demographic and socioeconomic factors—reflected in diet and other social determinants of health—that render the elderly, racial and ethnic minorities, and those with lower socioeconomic status more likely to suffer worse outcomes from COVID-19 infection; these same groups have existing pathologies that correlate with dysbiosis of gut microbiota (22).
Recent studies in small groups of COVID-19 patients have identified major dysbiosis of the intestinal microbiome, with enrichment by opportunistic bacterial (Coprobacillus, Clostridium species) and fungal pathogens (Candida, Aspergillus species), and depletion of beneficial symbionts (Faecalibacterium) that are positively and inversely correlated with COVID-19 severity (23, 24). In addition, an inverse correlation was noted between abundance of Bacteroides and SARS-Co-V2 load in fecal material during the course of hospitalization of these patients. Recently, there have been reports of viable virus particles in the stool (25, 26), although the significance and impact of these viral particles on the gut microbiome and infection transmission is not known. Recent demographic analyses of COVID-19−associated mortality rates (death/106) in 122 countries have suggested that inadequate sanitation and exposure to microbial diversity (including Gram-negative bacteria) may be associated with reduced COVID-19−associated mortality in developing and underdeveloped countries. The authors noted an inverse correlation between COVID-19−associated death rates and water quality scores, fraction of the population living in slums, and fraction with diarrhea. With these data, the authors proposed that microbially stimulated, innately enhanced levels of type I interferon (IFN) may be protective against COVID-19 mortality in these populations (27).
In addition to certain microbial taxa correlating with severity of COVID-19, several chronic conditions act as comorbidity factors for COVID-19, including cardiovascular disease and associated hypertension, diabetes, obesity, and asthma. Among these conditions, obesity, type 2 diabetes, and hypertension are the most important predisposing conditions for COVID-19 severe disease (28). Changes in the microbiome at times may modulate genetic susceptibility to these diseases in humans and animal models (29).
Recent data have shown that a major driver of the above-mentioned phenomena is the host immune system. The gut microbiome plays a major role in “training” the immune system, and changes in microbiome composition or activity may affect activity of several immune cell types (lymphoid, myeloid) (29). These effects may be mediated in part by direct exposure of developing immune cells in situ in the gut, or by the production of different microbial metabolites that can act in other organs distant from the gut. COVID-19 fatalities are often associated with an overwhelming and pathological inflammatory response in the lungs (30), caused by overproduction of proinflammatory cytokines (cytokine storm), as well as exhaustion of populations of immune cells (CD8+ T cells) (31). IFNs play an important role in the antiviral host response (32), which could be influenced by microbiome composition. Might differences in gut microbiome and associated immune cell programing influence individual host responses to SARS-CoV2 infection? Altering the microbiome composition through oral probiotics has been shown to alter the course and severity of other respiratory infections, such as influenza (33).
Finally, SARS-CoV2 infection may directly affect the gut and airway microbiomes. The cell surface receptor for the virus (ACE2, angiotensin converting enzyme 2) is expressed on airway epithelial cells and on enterocytes along the digestive tract. Studies are underway to characterize the respiratory microbiome and COVID-19 infections. Although the data are limited, the probiotics Lactobacillus and Bifidobacterium appear depleted in the intestines of COVID-19 patients (33), indicating an abnormal state (termed dysbiosis). Moreover, hospitalized COVID-19 patients may receive high-dose antibiotics, which dramatically alters microbial populations. More detailed knowledge of microbiota changes during COVID-19 awaits further results.
Hygienic Measures during a Pandemic and Their Effects on Microbiome Acquisition, Loss, and Reinoculation
“Hygiene” has long been associated with conditions and practices that promote health and prevent disease. Although hygiene is not the same as “sterilization,” it can shade into other practices that not only clean but can also reduce microbial load, as a review of hand hygiene has noted (34). Hygiene is of crucial importance in keeping people across the globe healthy. That said, the COVID-19 pandemic has provoked radical and immediate changes in hygienic measures, especially in high-resource countries, at individual and societal levels. Measures include deployment of personal protective equipment for health workers and certain essential workers, extensive use of surgical masks, frequent handwashing and application of hand sanitizer, and continuous cleaning with bleach in public areas and with disinfectants in homes. Cleaning is essential in high-density public locations, but we need more social investigations of the array of home cleaning practices, which are likely undertaken together. From a microbial perspective, these measures may affect microbiome transmission (Table 1). Prior to the COVID-19 pandemic, some public recognition of these insights in high-resource countries seemed to be loosening hygienic practices and accepting exposure to varied microbes (35).
Implementing much stricter hygienic practices now to contain COVID-19 transmission is necessary, but increased hygiene may come at a microbial cost by decreasing microbial acquisition and reinoculation following loss, although that cost is not yet known. For wealthier populations that can strictly adhere to hygienic measures in this pandemic, this cost may compromise useful microbiota functions. How hygiene measures affect the microbiome is a crucial research question. If loss of microbiome diversity occurs, and potentially even microbial extinction, could these microbial changes ultimately affect rates of asthma, obesity, or diabetes and other diseases that have microbial links? More critically, are there possible measures that might be taken during the pandemic to counterbalance the potential damage to the microbiome and ultimately catalyze other diseases associated with microbiome shifts? Over time, fear of pathogenic microbes can be balanced with a more nuanced attitude recognizing that taxonomically diverse microbiomes are known to strengthen immune systems and provide other benefits.
At the same time, the consequences of COVID for hygienic practices, and by implication microbiomes, differ across the planet. The World Health Organization (WHO) and UNICEF estimated in 2019 that one in three people around the world do not have access to safe drinking water, and that at least 2 billion people use water sources contaminated with feces (36). Moreover, a recent investigation in 16 sub-Saharan African countries found that the poorest households have serious difficulties gaining access to soap and water for washing (alcohol-based solutions are out of the question), and only 33.5% of households with a handwashing site had water and soap (37). An astonishing 60% of the world’s population (4.5 billion) has no access to safe sanitation, according to the WHO/UNICEF Joint Monitoring Program for Water Supply, Sanitation and Hygiene. Although these shortfalls are concentrated in LMICs, even the wealthiest countries contain populations with limited or no access to clean water and good sanitation systems. Intensifying hygienic practices may have little relevance for a sizeable proportion of the world’s people without the means to follow recommended hygienic practices to prevent COVID-19 transmission.
A potential consequence of COVID-19 across wealthy countries and LMICs is the use of antimicrobial treatments because of misdiagnoses, treatment of secondary infections from COVID, or self-medication (38). Early in the pandemic, hydroxychloroquine, which has a lengthy history in Africa as an antimalarial, was proposed as a possible COVID treatment. With the onset of the global pandemic and the publication of a widely viewed video in France and West Africa, West African pharmacies and street sellers experienced skyrocketing demand for chloroquine and hydroxychloroquine (39). Use of this drug to treat or prevent COVID has continued in 2020 (40, 41). The drug has also been shown to affect substantially gut microbiota (42). More generally, increased use of antimicrobials during this pandemic (especially during the early stages of the outbreak) may affect human microbiomes across the globe, although its effects remain unknown.
Feeding Ourselves and Our Microbiome during a Pandemic
The COVID-19 pandemic and control measures have also affected how the world’s populations are eating, although in different ways, and with varied consequences for the gut microbiome, human physiology, and health. These altered dietary practices have resulted from disrupted food supply chains. Decreased global trade, ruptured transportation networks, infection of food workers, runs on specific foodstuffs, privateering, closures of restaurants and food outlets, and increased home cooking have all exerted huge stress on food supply chains at a time of increased demand (43). The pandemic has thus profoundly transformed food access and consumption patterns in a short time. Depending on their economic status and location, some people have increasingly relied on food retailers that provide locally available foodstuffs (44). For some of those working from home, snacking and eating frequency may have increased (45). Closures of school cafeterias, decreased physical activity, and stress-related consumption of processed, unhealthy foods seems to be fueling obesity during the pandemic (46). Lockdowns and restaurant, café, and takeaway closures have also encouraged dietary improvements through home cooking and reduced processed food consumption in some locations and among some demographic groups (47).
Vulnerable populations include those facing food insecurity from the economic effects of pandemic response, predisposing conditions to COVID-19, and experiencing war, or other types of pest and disease outbreaks. The Food and Agriculture Organization expects that, for vulnerable groups facing these challenges, COVID-related lockdowns, economic declines, and uneven recoveries will exacerbate hunger and malnutrition: These populations simply will not have the income to gain access to food, or, elsewhere, may have to rely on expensive packaged and processed foods (43). Foods available through food banks and donation programs, and sometimes even schools, rarely meet the dietary needs of people with conditions like obesity, diabetes, and hypertension, and may even exacerbate them (48).
These changes in food consumption timing, quantity, quality, and frequency may profoundly impact gut microbiome composition and function. At the physiological level, pandemic-induced changes in dietary patterns may influence nutrients available to gut microbiota, possibly tipping the balance from beneficial toward detrimental gut bacterial functions and potentially contributing to intestinal inflammation and a host of chronic diseases (49). To be sure, for specific populations, consumption of healthier, fiber-rich diets may favor a better balance of gut bacteria and greater resistance to the coronavirus (50). Understanding the microbial consequences of these COVID-19−induced dietary shifts and developing interventions, particularly for infants and children, is important. It will be even more critical to do so in vulnerable populations who do not have access to enough or sufficiently nutritious diets during and after the pandemic. Chronic malnutrition and stunting among sub-Saharan African children is associated with gastrointestinal tract bacterial “decompartmentalization,” so that oropharyngeal bacteria are displaced to pathways from the stomach to the colon (51). Such markers are associated with lifelong health problems, from susceptibility to further infection to psychomotor developmental delays. Moreover, a recent Lancet Commission underscored the overlap of malnutrition and obesity, increasingly a problem in LMICs (52). For many populations, then, COVID-19 dietary changes may be exacerbating these already serious conditions associated with microbiome-related dysbiosis.
Social Microbiomes
Social interactions, including mobility, are key contributors to gut microbiota composition (53) and affect human health (Fig. 1). What have been considered “noncommunicable diseases” (obesity, diabetes) have important microbial causality (1). Microbial transmission, then, may be facilitated by shared social practices and interactions. COVID-19 control efforts—including isolation, physical distancing, the implementation of social bubbles, mobility restrictions, and border closures—have all disrupted or transformed social interactions and mobility patterns associated with microbial transfer, and could potentially have a significant impact on human microbiomes.
The COVID-19 pandemic response has entailed restrictions on an unprecedented scale, although most control measures are not new. Broadly denoting restrictions on movement of people, animals, and goods to curtail infectious disease, quarantine first emerged in Dubrovnik in the late 14th century. It eventually comprised multiple measures (sanitary cordons, isolation, lazarettos, restrictions, and sometimes reprisals against those seen as responsible for the epidemic) imposed over time to curtail plague and, in subsequent centuries, to limit smallpox, cholera, and yellow fever, and, more recently, Ebola and SARS transmission (54). COVID-19 measures around the world draw from this long history of social and mobility constraints. State-imposed constraints, self-imposed isolation, and socializing in “social bubbles” lead to fewer social contacts; the result may be microbiomes that resemble those of other household members or socializing partners (55).
The pandemic therefore offers an opportunity to examine the diverse consequences of reduced social contacts, isolation, and physical distancing on human microbiomes. Even within a household, the consequences of these social measures may be multiple. For working adults, shift work affects the microbiome via circadian rhythms (56), so would restructuring of day and nighttime routines influence those remaining at home in isolation? Does isolation—individual, with families, or in social bubbles—reduce diversity of the microbiome? How might stress and anxiety borne of social isolation influence the microbiome (57)? What might be the gendered consequences of lockdown and stress for the microbiome within households? Although men are more likely to die from COVID-19, women suffer disproportionately the secondary effects of this pandemic: They face greater income insecurity as lower-wage, part-time workers, are often home caregivers, and may be subject to domestic violence, and their needs for sexual and reproductive health tend to be deferred (58).
Not all people are able to implement physical distancing for extended periods, and it would be important to examine the consequences of their living and working conditions for the microbiome. Essential workers, including health workers and laborers in meat and poultry processing plants, must go to their workplaces and carry out duties under hazardous conditions (59). Those living in crowded, poor neighborhoods in LMICs and wealthy countries across the planet also face enormous difficulties adhering to lockdowns, physical distancing, and isolating the sick; investigations ranging from Dakar to Detroit reveal important intersections between race, poverty, and difficulties of physical distancing (60). Not only do workers, racial and ethnic minorities, and other at-risk populations tend to live in households with family members suffering from preexisting conditions and thus at high risk for COVID-19 morbidity and mortality, but they frequently have less access to health care, and, in some countries, have no health insurance (61). Is it possible under such circumstances to communicate a dysbiotic microbiome to those living in close proximity? If so, what possible measures could be taken to mediate the impacts on the microbiome?
COVID-19 restrictions to limit short-distance movements, reduced train and car travel, shuttered airports, and border closure will limit diverse environmental exposures and likely impact human microbial diversity during multiple pandemic waves and their aftermath. Hence, examining the variable effects of COVID-19 mobility restrictions on human microbiomes is an important research question, but depends, in part, on implementation and experiences of these restrictions. Implementation of these measures has varied substantially across countries. In the winter and spring of 2020, stricter controls were imposed in, for instance, China, Italy, and France, whereas fewer controls were put in place elsewhere, including Niger, Japan, Tanzania, Sweden, and some US states.
Another consideration is how these restrictions affect preexisting microbiome diversity among different populations. Populations with differential use of and exposure to disinfecting products, consumption of healthy or processed foods, and access to outdoors would clearly experience diverse impacts on their microbiota (62). Gut microbial composition differs across the world’s populations, with highest diversity observed among people living in more isolated rural settings, consuming high quantities of fibers and very little or no sugar, processed foods, or antimicrobials (63, 64).
Mobility—from globe-crossing business travelers to pastoralists herding their cattle to graze in seasonal pasturelands—can offer exposure to microbial species that may be missing in one’s microbiome (65). Travel restrictions imposed to prevent COVID transmission might be a missed opportunity, and lost diversity may not be easily recoverable (66). Nonetheless, long-distance travelers also risk acquiring antimicrobial drug-resistant bacteria in their journeys, and migrants from certain settings have experienced rapid declines in microbial diversity (67, 68). How different kinds of movement affect the microbiomes of different social groups and populations merits additional attention.
Impacts of Hygiene on Microbiomes of the Young and the Old
Early and later life constitute critical periods when the microbiome exerts particularly important influences on health, translating into lifelong health and disease concerns and potential disparities in mortality and morbidity. COVID-19 has significantly, although differentially, affected children and seniors. Its influences on the microbiomes of young and old should be further investigated and addressed.
Early Life/Infants.
Birth and early infancy are critical periods for microbiome establishment and development. Newborns are colonized by maternal microbes acquired during vaginal delivery and through skin-to-skin contact, and their microbiomes are supported by prebiotic oligosaccharides and microbes provided in breast milk (69). Research in model animals has shown that the maternal immune system shapes the “healthy” microbiome of early life, and that this strategy spans across the animal kingdom (70). Moreover, a functionally and taxonomically diverse microbiome is key for infant immune system development. Yet, across the planet, early life microbiomes are changing due to increased antibiotics, Caesarean section rates, and formula feeding, and, among wealthy populations, increased hygiene as well as indoor and screen time. Being born by Caesarean section, for instance, increases the risk of later allergy, asthma, and obesity rates (71) through mechanisms that appear to be mediated, at least in part, by microbiome dysbiosis during infancy.
The effects of COVID-19 among mothers on pregnancy outcomes remain unclear, although vertical transmission in severe cases has been reported (72–74). We know even less, however, about how efforts to control COVID transmission affect an infant’s microbiome during this critical developmental period. Altered hospital, healthcare, and home care practices for infants and children can interrupt the “seeding and feeding” of their microbiomes. These changes merit further investigation. Higher Caesarean section rates may result from lower tolerance for transmission risks (75), and more home births may be a consequence of desires to avoid hospital exposure during the pandemic. Despite guidance from the WHO to support immediate postpartum mother−infant contact and breastfeeding, including for mothers with COVID-19 (using appropriate respiratory precautions), some hospitals have implemented infection prevention and control policies that impose separation and discourage or prohibit breastfeeding (76). Other jurisdictions are promoting early hospital discharge for healthy dyads and suspending postpartum home visits by public health nurses, thereby limiting lactation support for new mothers. Although data are still emerging, current evidence suggests that SARS-CoV2 transmission via breastfeeding is unlikely (77). For nonbreastfed infants, formula hoarding/shortages may also affect feeding practices.
At home, increased sanitization practices and limited social interactions could reduce infant contact with “normal” environmental microbes. Stress caused by the pandemic could alter the maternal or caregiver microbiome, which could be transmitted to the infant. Infants are falling behind in their regular vaccination schedule, either because caregivers want to avoid health care structures or because health services are overwhelmed (78). Some families may avoid emergency rooms and health care visits, and potentially not receive needed treatments, thereby affecting the microbiome through, for instance, diarrheal or vaccine-preventable diseases. Young children who normally attend school may be sequestered at home, significantly decreasing their contacts with others. The state of the home (e.g., fastidious cleaning using disinfectants, part of an array of other cleaning practices), animal exposure, and access to outdoors may also shape the child’s microbiome. Conversely, it is possible that mothers working from home may permit them to sustain breastfeeding longer than would otherwise be possible, and some families may spend more time outdoors or with pets, increasing contact with “healthy microbes.”
One major unanswered question concerns the long-term impact of COVID-19 infection on the early life microbiome. Infant and childhood mortality due to COVID-19 is extremely low, but the effects of asymptomatic carriage on the infant microbiome are unknown.
Later Life/Elderly.
The most recent statistics on the first wave of SARS-CoV2 infection indicate that the vast majority of severe COVID-19 cases occur in the elderly, with people over age 70 accounting for >90% of mortality (79). Although higher mortality might result from generally frail health status or living in transmission-prone settings, the microbiome decreases in diversity among the elderly (80) and may play a potential role in COVID-19 severity and mortality. Most chronic diseases associated with aging have some link to the microbiome (81).
COVID-19 has dramatically affected the care, mobility, and social interactions of elder populations. Hospitalized seniors or those living in institutional settings have experienced limited or no contact with family members, with consequences for their mental and physical health. Seniors may have greater anxiety and fear for their own health during this pandemic, but must simultaneously cope with the absence of social support and physical and emotional connection, for their own and others’ health. Isolation from family members is generally associated with poor prognosis (82). This isolation is not only social but has important sensorial dimensions—just as important at the end of life as it is in the beginning. Anthropologists have emphasized the importance of touch, which may have critical implications for reinoculation of missing microbes from younger to older generations (83).
Are relatively high rates of COVID-19 disease and severity among people living in institutionalized settings due primarily to viral exposure, or could there be microbiome-mediated risk? Such investigation should explore whether the elderly living in institutionalized settings have a more impoverished microbiome compared to those living at home with extended families. A host of factors shape aging people’s microbiome: dietary differences; institutional use of sanitizing products; different social exchange of microbiome with similarly aged people in institutions compared to younger people (e.g., grandchildren at home); and multiple medicine use, including antibiotics and other antimicrobials to manage preexisting chronic illnesses (84).
The microbiomes of aging people are also influenced by communal interactions, leading us to wonder whether the sequestering of care for the elderly and chronically ill have created the conditions that facilitate viral transmission. Residing in variously sized built environments, the elderly interact with coresidents suffering from comorbidities and engaging in high medication use, as well as care providers. Does this form of community dysbiosis contribute to high rates of mortality in care homes, especially if there is little exposure to healthy family members, the outdoors, and a healthy diet?
Elsewhere in low-income countries, where the elderly more likely live in multigenerational households, we also wonder what impact varied social contacts and interactions might have on their microbiomes and on COVID-19 infection. Would these interactions outweigh other chronic health conditions, including low nutritional status (85)? At present, we do not have sufficient data to answer these important questions.
The Communal Microbiome
COVID-19 sharpens our focus on how preventive health practices, such as antimicrobial use, may have collateral damage on a collective microbiome. Physical distancing and increased hygiene measures reduce COVID-19 infections, but may also indirectly reduce the general microbial reservoir and its transmission for some, although not all, populations around the world. Populations able to isolate from each other and to disinfect their living and work spaces reduce their exposure to social and environmental microbial pools; they are thus more likely to experience changes in their microbiomes (86). These dynamics will likely further diminish collective microbial diversity, increasing dysbiosis in microbial function, altered immune function, and, possibly, chronic inflammation. An insult to the microbiome through, for instance, antibiotic use, compounded by an already depleted collective microbial reservoir, will make it more difficult to reconstitute a healthy microbiome. The consequence may be heightened susceptibility to infection, more severe symptoms, and greater mortality.
We want to be clear: Preventing COVID-19 transmission is necessary, and the hygienic transformations of the past 100 years have resulted in major reductions in mortality from infectious diseases. But the intersection of the past century’s hygienic practices and recent COVID-19 pandemic control measures may negatively affect the microbiome and thus human health across multiple timescales. As morbidity and mortality increase in relation to these microbial changes, human evolutionary trajectories may also change. Studies in mice, for instance, have shown that once particular microbial taxa are lost from a population over generations, they are difficult to recover (66). The associated loss of microbial function can severely limit host ability to survive in certain environments or to resist infections (66). A fundamental question, then, is what microbial functions might we lose as a result of COVID-19 prevention efforts? What are the consequences as humans continue to encounter nutritional and immune challenges in future generations, and what can be done to mitigate them (Table 1)?
It is worth considering how to deploy physical distancing and hygiene practices to prevent COVID-19 transmission, but also to sustain and protect diversity of the microbiome. It is important to understand more fully how these practices affect the microbiome, and then, in response, to develop public measures and practices that can, if appropriate, increase exposure to beneficial microbes and simultaneously reduce risk of COVID-19 transmission. Public measures could include those already associated with healthy microbial diversity: keeping open urban parks but ensuring the maintenance of physical distancing; offering remote support for breastfeeding mothers and encouraging infant vaccination; and ensuring the provision of healthy food assistance to low-income families and children. Individual practices could include safely spending time outdoors, gardening where possible, eating a fiber-rich diet, avoiding unnecessary antibiotics, and encouraging physical contact among coquarantined family members and pets, all of which have been shown to facilitate retention and transmission of beneficial microbes. In LMICs, expanding access to clean water and soap and masks, tackling food insecurity, and reducing easy access to antibiotics may effectively reduce transmission; we hypothesize that these measures could also sustain microbial variability (87, 88). Our knowledge of COVID-19 and the microbiome is incomplete, and we have only begun to explore their interactions. Nevertheless, these suggested measures and practices could prevent COVID transmission, simultaneously reduce the negative impacts of pandemic control measures on human microbiomes, and, potentially, offer important health benefits for future generations.
Conclusion
The current pandemic has disrupted the world as we knew it. Despite the damage and turmoil that COVID-19 has already caused worldwide, it also reminds us that we live in a microbial world where microbes have a major impact on all facets of our existence. This pandemic presents a significant opportunity to study, in real time, the relationship between an infectious agent, the microbiome, precipitous and uneven social and economic changes, and their combined effects on health and disease. As we track changes in the microbiome during COVID-19, we can apply this knowledge to current pandemic control measures and recovery. These insights and new measures will provide a platform to improve our management of the next pandemic disruption.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data Availability.
All study data are included in the article.
References
- 1.Finlay B. B.; CIFAR Humans; Microbiome , Are noncommunicable diseases communicable? Science 367, 250–251 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Strachan D. P., Hay fever, hygiene, and household size. BMJ 299, 1259–1260 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scudellari M., News feature: Cleaning up the hygiene hypothesis. Proc. Natl. Acad. Sci. U.S.A. 114, 1433–1436 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stiemsma L. T., Reynolds L. A., Turvey S. E., Finlay B. B., The hygiene hypothesis: Current perspectives and future therapies. ImmunoTargets Ther. 4, 143–157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Bastard Q., et al., Systematic review: Human gut dysbiosis induced by non-antibiotic prescription medications. Aliment. Pharmacol. Ther. 47, 332–345 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Sonnenburg J. L., Sonnenburg E. D., Vulnerability of the industrialized microbiota. Science 366, eaaw9255 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Bokulich N. A., et al., Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 8, 343ra382 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaser M. J., Falkow S., What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol. 7, 887–894 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rook G. A., Hygiene and other early childhood influences on the subsequent function of the immune system. Dig. Dis. 29, 144–153 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Buford T. W., (Dis)trust your gut: The gut microbiome in age-related inflammation, health, and disease. Microbiome 5, 80 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahenzli J., Köller Y., Wyss M., Geuking M. B., McCoy K. D., Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 14, 559–570 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohnmacht C., et al., MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORγt+ T cells. Science 349, 989–993 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Illi S.et al.; GABRIELA Study Group , Protection from childhood asthma and allergy in Alpine farm environments—The GABRIEL Advanced Studies. J. Allergy Clin. Immunol. 129, 1470–7.e6 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Aguirre A. A., Changing patterns of emerging zoonotic diseases in wildlife, domestic animals, and humans linked to biodiversity loss and globalization. ILAR J. 58, 315–318 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Denyer Willis L., Chandler C., Quick fix for care, productivity, hygiene and inequality: Reframing the entrenched problem of antibiotic overuse. BMJ Glob. Health 4, e001590 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giles-Vernick T., Bainilago L., Fofana M., Bata P., Vray M., Home care of children with diarrhea in Bangui’s therapeutic landscape (Central African Republic). Qual. Health Res. 26, 164–175 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Williams D. R., Mohammed S. A., Leavell J., Collins C., Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities. Ann. N. Y. Acad. Sci. 1186, 69–101 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb J. L. A. J., The Guts of the Matter: A Global History of Human Waste and Infectious Intestinal Disease (Studies in Environmental History, Cambridge University Press, Cambridge, United Kingdom, 2019). [Google Scholar]
- 19.Wade L., An unequal blow. Science 368, 700–703 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Napier A. D., Fischer E. F., The culture of health and sickness. Le Monde Diplomatique, 4 July 2020. https://mondediplo.com/2020/07/04uganda. Accessed 4 July 2020.
- 21.Trottein F., Sokol H., Potential causes and consequences of gastrointestinal disorders during a SARS-CoV-2 infection. Cell Rep. 32, 107915 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raisi-Estabragh Z., et al., Greater risk of severe COVID-19 in Black, Asian and minority ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: Study of 1326 cases from the UK biobank. J. Public Health 42, 451–460 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo T., et al., Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 159, 944–955.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scaldaferri F., et al., The thrilling journey of SARS-CoV-2 into the intestine: From pathogenesis to future clinical implications. Inflamm. Bowel Dis. 26, 1306–1314 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamers M. M., et al., SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J., et al., Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 26, 1077–1083 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Kumar P., Chander B., COVID 19 mortality: Probable role of microbiome to explain disparity. Med. Hypotheses 144, 110209 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J., et al., Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin. Microbiol. Infect. 26, 767–772 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeyakumar T., Beauchemin N., Gros P., Impact of the microbiome on the human genome. Trends Parasitol. 35, 809–821 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Yang Y., et al., Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv:2020.03.02.20029975 (6 March 2020).
- 31.Diao B., et al., Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). medRxiv:2020.02.18.20024364 (20 February 2020). [DOI] [PMC free article] [PubMed]
- 32.Park A., Iwasaki A., Type I., Type I and type III interferons—Induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 27, 870–878 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mak J. W. Y., Chan F. K. L., Ng S. C., Probiotics and COVID-19: One size does not fit all. Lancet Gastroenterol. Hepatol. 5, 644–645 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandegrift R., et al., Cleanliness in context: Reconciling hygiene with a modern microbial perspective. Microbiome 5, 76 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodgetts T., et al., The microbiome and its publics: A participatory approach for engaging publics with the microbiome and its implications for health and hygiene. EMBO Rep., 19, e45786 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization , 1 in 3 people globally do not have access to safe drinking water. https://www.who.int/news/item/18-06-2019-1-in-3-people-globally-do-not-have-access-to-safe-drinking-water-unicef-who. Accessed 29 July 2020.
- 37.Jiwani S. S., Antiporta D. A., Inequalities in access to water and soap matter for the COVID-19 response in sub-Saharan Africa. Int. J. Equity Health 19, 82 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rawson T. M., Ming D., Ahmad R., Moore L. S. P., Holmes A. H., Antimicrobial use, drug-resistant infections and COVID-19. Nat. Rev. Microbiol. 18, 409–410 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Desclaux A., La mondialisation des infox et ses effets sur la santé en Afrique: L’exemple de la chloroquine. The Conversation, 19 March 2020. https://theconversation.com/la-mondialisation-des-infox-et-ses-effets-sur-la-sante-en-afrique-lexemple-de-la-chloroquine-134108. Accessed 14 June 2020.
- 40.Abena P. M., et al., Chloroquine and hydroxychloroquine for the prevention or treatment of COVID-19 in Africa: Caution for inappropriate off-label use in healthcare settings. Am. J. Trop. Med. Hyg. 102, 1184–1188 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belayneh A., Off-label use of chloroquine and hydroxychloroquine for COVID-19 treatment in Africa against WHO recommendation. Res. Rep. Trop. Med. 11, 61–72 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maier L., et al., Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galanakis C. M., The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods 9, 523 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hobbs J. E., Food supply chains during the COVID-19 pandemic. Canadian J. Agric. Econ. 68, 171–176 (2020). [Google Scholar]
- 45.Zarrinpar A., Chaix A., Yooseph S., Panda S., Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 20, 1006–1017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rundle A. G., Park Y., Herbstman J. B., Kinsey E. W., Wang Y. C., COVID-19-related school closings and risk of weight gain among children. Obesity (Silver Spring) 28, 1008–1009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Renzo L., et al., Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 18, 229 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ippolito M. M., et al., Food insecurity and diabetes self-management among food pantry clients. Public Health Nutr. 20, 183–189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy M., Kolodziejczyk A. A., Thaiss C. A., Elinav E., Dysbiosis and the immune system. Nat. Rev. Immunol. 17, 219–232 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Rodríguez-Pérez C., et al., Changes in dietary behaviours during the COVID-19 outbreak confinement in the Spanish COVIDiet study. Nutrients 12, 1730 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vonaesch P.et al.; Afribiota Investigators , Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proc. Natl. Acad. Sci. U.S.A. 115, E8489–E8498 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Popkin B. M., Corvalan C., Grummer-Strawn L. M., Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 395, 65–74 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasquaretta C., Gómez-Moracho T., Heeb P., Lihoreau M., Exploring interactions between the gut microbiota and social behavior through nutrition. Genes (Basel) 9, 534 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tognotti E., Lessons from the history of quarantine, from plague to influenza A. Emerg. Infect. Dis. 19, 254–259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brito I. L., et al., Transmission of human-associated microbiota along family and social networks. Nat. Microbiol. 4, 964–971 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mortaş H., Bilici S., Karakan T., The circadian disruption of night work alters gut microbiota consistent with elevated risk for future metabolic and gastrointestinal pathology. Chronobiol. Int. 37, 1067–1081 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Malan-Muller S., et al., The gut microbiome and mental health: Implications for anxiety- and trauma-related disorders. OMICS 22, 90–107 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Wenham C., Smith J., Morgan R.; Gender and COVID-19 Working Group , COVID-19: The gendered impacts of the outbreak. Lancet 395, 846–848 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dyal J. W., et al., COVID-19 among workers in meat and poultry processing facilities - 19 states, April 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 557−561 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Corburn J., et al., Slum health: Arresting COVID-19 and improving well-being in urban informal settlements. J. Urban Health 97, 348–357 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hawkins D., Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity. Am. J. Ind. Med. 63, 817–820 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilbert J. A., Stephens B., Microbiology of the built environment. Nat. Rev. Microbiol. 16, 661–670 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Wilson A. S., et al., Diet and the human gut microbiome: An international review. Dig. Dis. Sci. 65, 723–740 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clemente J. C., et al., The microbiome of uncontacted Amerindians. Sci. Adv. 1, e1500183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosites E., et al., Microbiome sharing between children, livestock and household surfaces in western Kenya. PLoS One 12, e0171017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sonnenburg E. D., et al., Diet-induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voor in ‘t holt A. F., et al., Acquisition of multidrug-resistant Enterobacterales during international travel: A systematic review of clinical and microbiological characteristics and meta-analyses of risk factors. Antimicrobial Resis. Infect. Control 9, 71 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vangay P., et al., US immigration westernizes the human gut microbiome. Cell Host Microbe 175, 962–972 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fehr K., et al., Breastmilk feeding practices are associated with the co-occurrence of bacteria in mothers’ milk and the infant gut: The CHILD Cohort Study. Cell Host Microbe 28, 285–297.e4 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Furman O., et al., Stochasticity constrained by deterministic effects of diet and age drive rumen microbiome assembly dynamics. Nat. Commun. 11, 1904 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keag O. E., Norman J. E., Stock S. J., Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis. PLoS Med. 15, e1002494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Juan J., et al., Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: Systematic review. Ultrasound Obstet. Gynecol. 56, 15–27 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alberca R. W., Pereira N. Z., Oliveira L. M. D. S., Gozzi-Silva S. C., Sato M. N., Pregnancy, viral infection, and COVID-19. Front. Immunol. 11, 1672 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galang R. R., et al., Severe coronavirus infections in pregnancy: A systematic review. Obstet. Gynecol. 136, 262–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qi H., et al., Safe delivery for pregnancies affected by COVID-19. BJOG 127, 927–929 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Tomori C., Gribble K., Palmquist A. E. L., Ververs M.-T., Gross M. S., When separation is not the answer: Breastfeeding mothers and infants affected by COVID-19. Maternal Child Nutrition 16, e13033 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tun M. H.et al.; CHILD Study Investigators , Postnatal exposure to household disinfectants, infant gut microbiota and subsequent risk of overweight in children. CMAJ 190, E1097–E1107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bramer C. A., et al., Decline in child vaccination coverage during the COVID-19 pandemic—Michigan Care Improvement Registry, May 2016-May 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 630–631 (2020). [DOI] [PubMed] [Google Scholar]
- 79.Li X., et al., Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 146, 110–118 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bana B., Cabreiro F., The microbiome and aging. Annu. Rev. Genet. 53, 239–261 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Zapata H. J., Quagliarello V. J., The microbiota and microbiome in aging: Potential implications in health and age-related diseases. J. Am. Geriatr. Soc. 63, 776–781 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holt-Lunstad J., Smith T. B., Baker M., Harris T., Stephenson D., Loneliness and social isolation as risk factors for mortality: A meta-analytic review. Perspect. Psychol. Sci. 10, 227–237 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Geissler P. W., Prince R., The Land Is Dying: Contingency, Creativity and Conflict in Western Kenya (Berghahn, Oxford, United Kingdom, 2010). [Google Scholar]
- 84.Salazar N., Valdés-Varela L., González S., Gueimonde M., de Los Reyes-Gavilán C. G., Nutrition and the gut microbiome in the elderly. Gut Microbes 8, 82–97 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shahar S., Vanoh D., Mat Ludin A. F., Singh D. K. A., Hamid T. A., Factors associated with poor socioeconomic status among Malaysian older adults: An analysis according to urban and rural settings. BMC Public Health 19, 549 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller E. T., Svanbäck R., Bohannan B. J. M., Microbiomes as metacommunities: Understanding host-associated microbes through metacommunity ecology. Trends Ecol. Evol. 33, 926–935 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Brauer M., Bennitt F. B., Stanaway J. D., Global access to handwashing: Implications for COVID-19 control in low-income countries. Environ. Health Perspect. 128, 057005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maillard J. Y., et al., Reducing antibiotic prescribing and addressing the global problem of antibiotic resistance by targeted hygiene in the home and everyday life settings: A position paper. Am. J. Infect. Control 48, 1090–1099 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article.