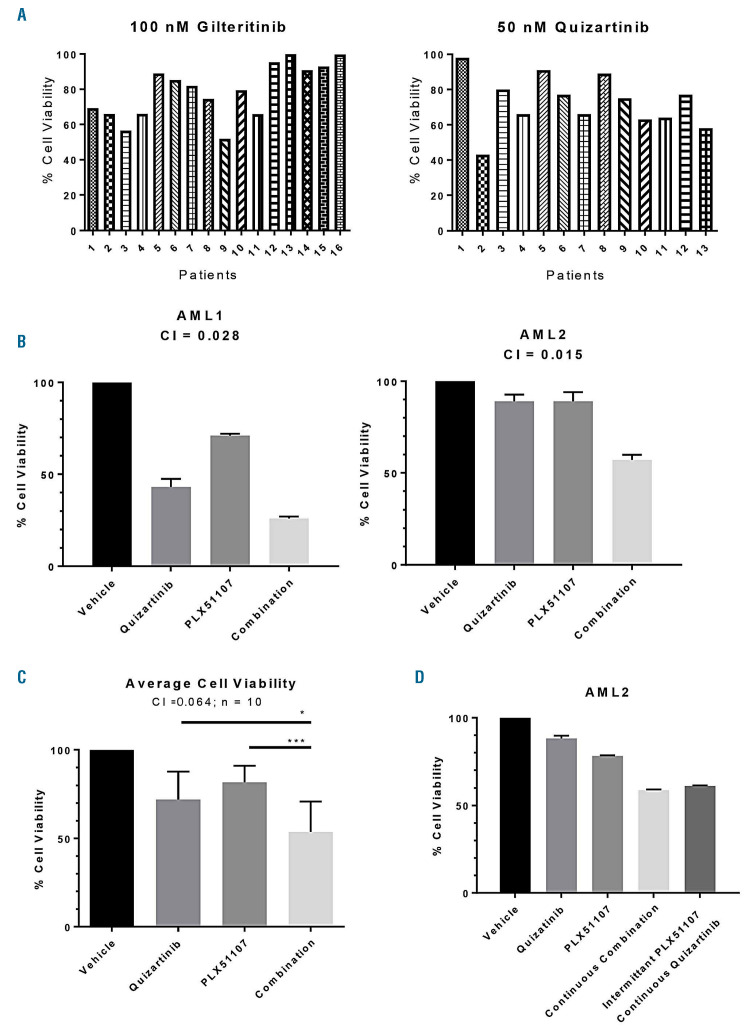
Figure 4.
Synergistic cytotoxicity of PLX51107 and quizartinib in primary acute myeloid leukemia samples on bone marrow stroma. (A) Primary blasts from patients with relapsed or refractory FLT3-ITD AML were co-cultured with BM stromal cells in the presence of the clinically achievable doses of either 100 nM gilteritinib (left) or 50 nM quizartinib (right) for 72 hours (h) and cell viability was assessed by a dimethyl-thiazole diphenol tetrazolium bromide (MTT) assay. Sample numbers in the gilteritinib series do not correspond to those in the quizartinib series, as they were assayed at different times. (B) Relapsed or refractory FLT3-ITD AML patients’ samples were co-cultured with BM stroma and treated with 50 nM quizartinib, 250 nM PLX51107, or a combination (50 nM quizartinib and 250 nM PLX51107) for 72 h prior to assessment for cell viability by MTT. Overall, a total of ten AML samples were screened. AML1 and AML2 are two representative cases. Patients' characteristics for AML1 and AML2 are provided in Online Supplementary Table S1. (C) Averages of each condition from the ten primary samples are shown, with standard deviations represented by error bars. *P<0.05, **P<0.01; two-tailed Student t-test. (D) Sample AML2 exposed to quizartinib and PLX51107 as in (B), but for this experiment, PLX51107 was washed out after 6 h exposure for each of the 3 days of exposure.