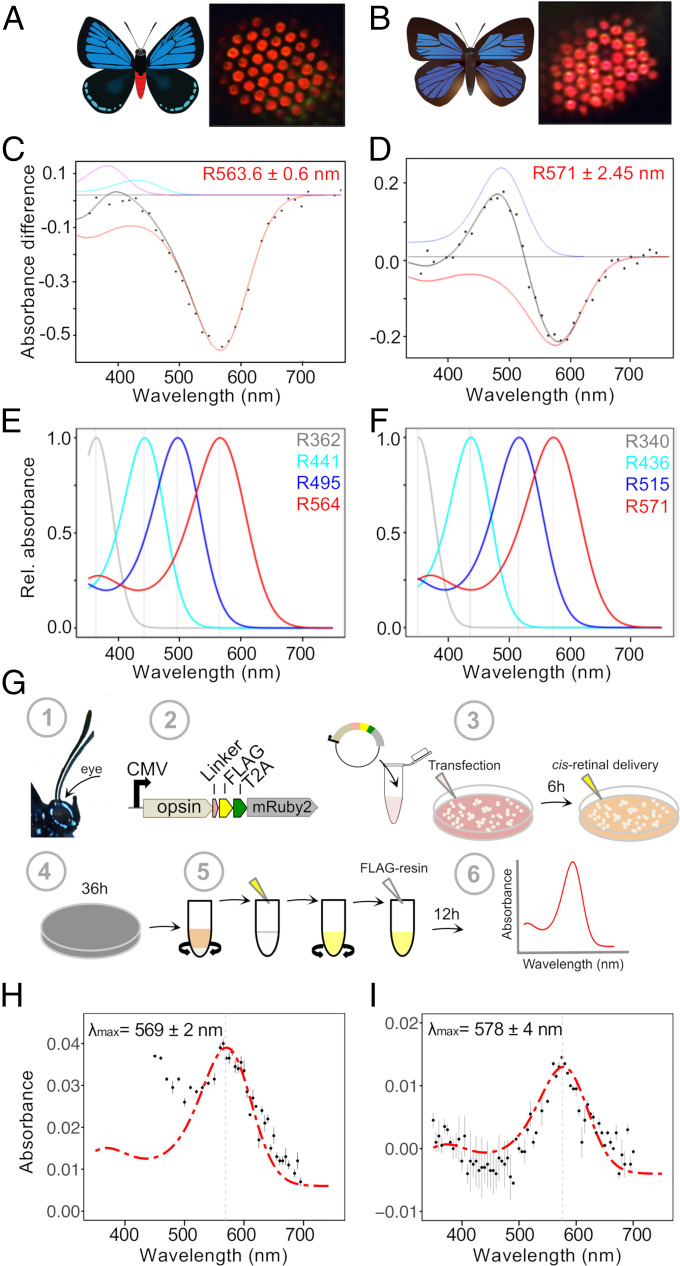
Fig. 2.
Red-shifted long-wavelength opsins extend spectral sensitivity toward the far-red in lycaenid butterflies. (A and B) Illustrations of E. atala (A) and A. japonica (B). Butterfly eyeshine photographs on the Right show a series of adjacent ommatidial units filled primarily with red rhodopsins in the dorsal retina (SI Appendix, Fig. S1). (C and D) Photochemical difference spectra (DS, black dots) were obtained following partial bleaches of long-wavelength rhodopsins from dorsal retina of intact butterflies and represent absorbance differences between amounts of rhodopsin (R, red) and metarhodopsin photoproducts when present (M, blue) after a dark period that followed photoconversion. Each black curve represents a computed difference spectrum for least square fits estimates at (C) R564 of E. atala and (D) R571 of A. japonica. In E. atala, the difference spectrum was acquired upon complete degradation of the M photoproduct and with small contributions from R440 (cyan) and retinal binding protein (RBP395, magenta). (E and F) Normalized eye spectral sensitivity of E. atala (E) and A. japonica (F) computed from a retinal densitometry analysis (SI Appendix, Fig. S2 I and J) reveals the contribution of four rhodopsins: UV, two blue opsins, and one long-wavelength opsin. (G) Schematics of the in vitro opsin purification assay. Opsin open reading frames (ORFs) were amplified from eye cDNA (1) and subcloned in an expression cassette derived from the pcDNA5 vector and containing a C-terminal FLAG epitope flanked by a peptide linker, a T2A cleavage site, and the cytoplasmic fluorescent mRuby2 marker for visualizing cell transfection efficiency (2). DNA–lipid complexes were transfected into HEK293T cells followed by 11-cis-retinal delivery. All subsequent steps were performed under dim red-light illumination (3). Culture plates were wrapped in aluminum foil and incubated for 36 h (4). Cells were harvested, active membrane-bound rhodopsins were extracted via membrane solubilization, and rhodopsin–chromophore complexes were nutated as detailed in Materials and Methods and SI Appendix (5). After an overnight incubation with FLAG resin, rhodopsin complexes were purified via FLAG resin affinity (6). All fractions were analyzed by Western blot, and the absorbance properties of the eluate fractions containing purified rhodopsins were measured via UV-VIS spectroscopy. (H and I) Functional characterization of red-shifted long-wavelength lycaenid butterfly opsins. Dark absorbance spectra of long-wavelength rhodopsin (LWRh) expressed and purified using the HEK293T transient cell culture system. LWRh absorbance spectra are indicated with black dots, and a rhodopsin template (136) was computed to obtain the best estimates of λmax fitting the data. (H) E. atala purified LW opsin with λmax = 569 nm, n = 2 protein eluate aliquots. (I) A. japonica purified LW opsin with λmax = 578 nm; n = 2 protein eluate aliquots. Bars represent ±SEs of the mean.