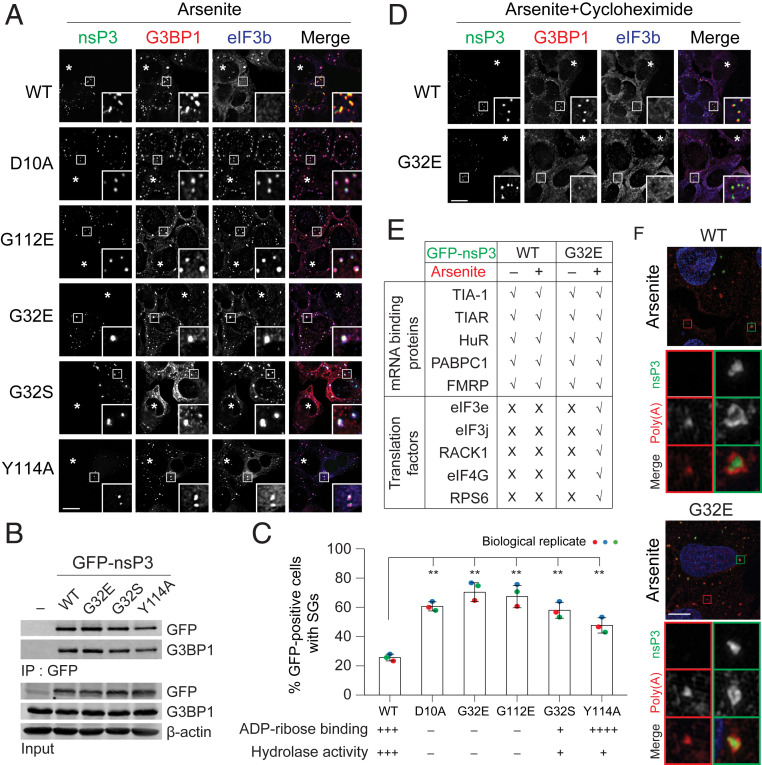
Fig. 3.
ADP-ribosylhydrolase activity of nsP3 suppresses SG formation. (A) U2OS cells transfected with GFP-tagged nsP3WT or different nsP3 point mutants (D10A, G32E, G32S, G112E, and Y114A) were treated with 0.2 mM arsenite for 30 min and immunostained for G3BP1 (red) and eIF3b (blue). Asterisks indicate untransfected cells. (B) 293F cells were transfected with either GFP vector or GFP-tagged nsP3WT, nsP3G32E, nsP3G32S, or nsP3Y114A. After 24-h transfection, cells were pelleted, lysed, immunoprecipitated using anti-GFP antibodies, and the immunoprecipitates were blotted against G3BP1 and GFP antibodies. (C) Bar graph shows the percentage of GFP-positive cells with SGs in A. (Lower) ADP-ribose binding and hydrolase activity of the mutants tested (31). **P < 0.01, two-tailed, unpaired Student’s t test. Error bars correspond to mean ± SD with n = 3. (D) Cells transfected with GFP-tagged nsP3WT or nsP3G32E were cotreated with 0.2 mM arsenite and 100 μg/mL cycloheximide for 30 min. Cells were then immunostained for G3BP1 (red) and eIF3b (blue). Asterisks indicate untransfected cells. (E) Table summarizes the colocalization of nsP3 (WT or G32E) with different mRNA binding proteins and translation factors as shown in SI Appendix, Fig. S3A. (F) Superresolution microscopy indicates how the distribution of poly(A)+ mRNA signal overlaps with nsP3 WT or G32E mutant. U2OS cells transfected with GFP-tagged nsP3WT or nsP3G32E were with 0.2 mM arsenite for 30 min and hybridized with oligo(dT) probes, followed by staining for GFP. Green and red boxes indicate the condensates from nsP3-transfected and untransfected cells, respectively. (Scale bars, 10 μm.)