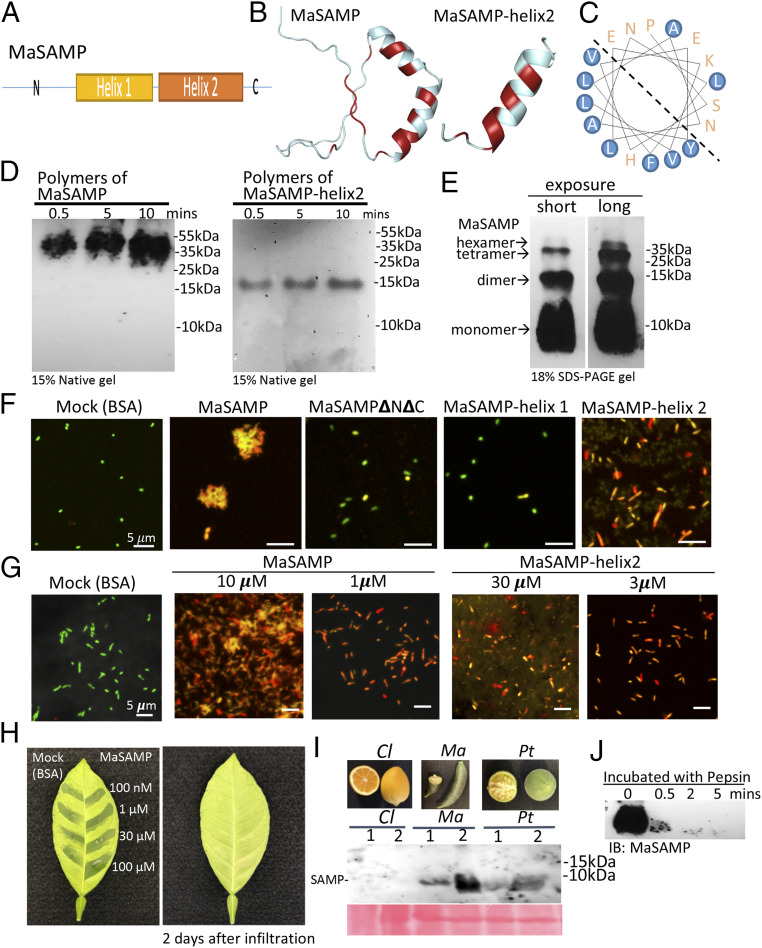
Fig. 5.
The α-helix2 domain of MaSAMP is the key bactericidal motif, and SAMP is present in fruits and rapidly degraded by pepsin. (A) The diagram of the SAMP structure. (B) The predicted structure of SAMP by the SWISS-MODEL. The hydrophobic residues are marked in red. (C) The helical wheel diagram of the α-helix2 domain was predicted. The hydrophobic residues are circled in blue. (D) MaSAMP and MaSAMP-helix2 domain form only polymers (likely hexamers) in the native PAGE gel. (E) MaSAMP forms SDS-resistant oligomers. (F and G) The bactericidal activity of various truncated MaSAMPs was examined using Lcr viability/cytotoxic assay. The green and red cells indicate the live and dead cells, respectively. (H) MaSAMP phytotoxicity was assessed by infiltrating different concentrations of MaSAMP or BSA solution into the leaf of sweet orange. (I) MaSAMP was detected by Western blot using the anti-MaSAMP antibody in the fruit tissue of Australian finger lime (Ma) and trifoliate orange (Pt) but not Lemon (Cl). The corresponding fruit pictures are shown in the upper panel. (J) MaSAMP was rapidly degraded after incubation with human pepsin over a time course.