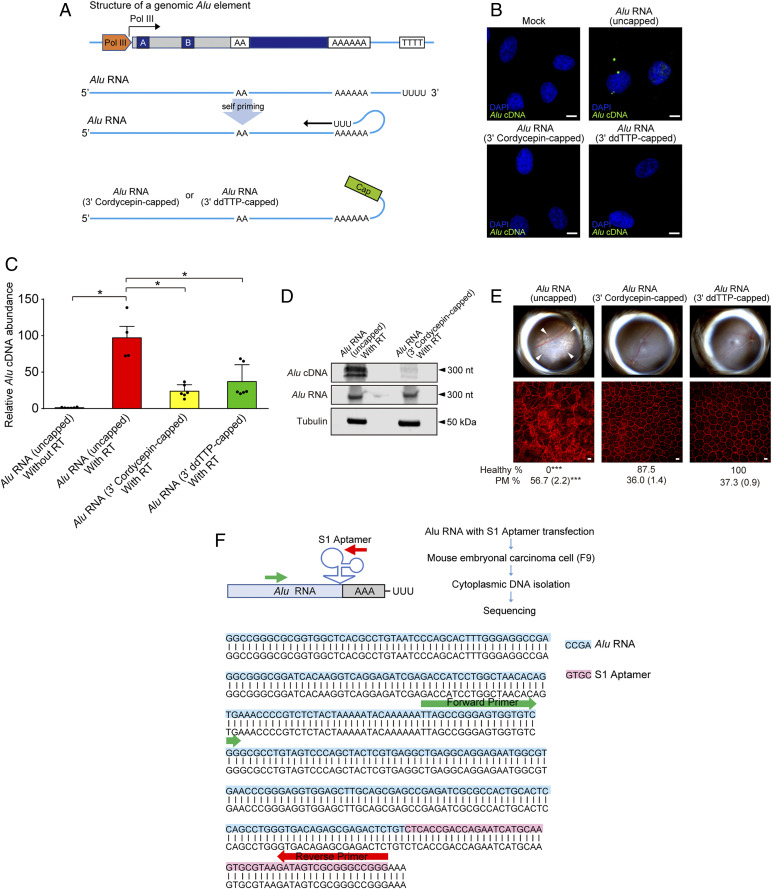
Fig. 4.
Alu cDNA is synthesized via self-priming. (A) Schematic of putative Alu RNA transcription and self-priming by 3′ complementary hybridization. Alu RNA was synthetically capped on the 3′ end via dideoxy thymidine base (ddTTP) or cordycepin triphosphate to prevent extension via RT. (B) In situ hybridization of Alu cDNA after transfection of uncapped and or 3′-capped Alu RNAs into WT mouse RPE cells. Green, Alu cDNA; blue, DAPI. Representative of n = 3 experiments. (Scale bar, 10 µm.) (C) Alu cDNA abundance in cytoplasmic fractions of WT mouse RPE cells via an ex vivo RT activity assay (SI Appendix, Supplementary Methods), in the absence of external primers, followed by Alu-specific real-time PCR. PCR was performed with Alu RNA that was uncapped, or 3′-capped with ddTTP or cordycepin triphosphate. *P < 0.05 by Mann–Whitney U test. (D) Blotting analysis of cytoplasmic fractions of WT mouse RPE cells to detect Alu cDNA, Alu RNA, and tubulin after performing an ex vivo RT activity assay in the absence of external primers (see above). Fractions were incubated with uncapped or 3′-cordycepin triphosphate-capped Alu RNA. (E) Fundus photographs (Top) and corresponding representative RPE sheet micrographs (Bottom) of WT mice administered uncapped Alu RNA or Alu RNAs capped on the 3′ end with the chain ddTTP or cordycepin triphosphate. (Scale bars, 10 μm.) The arrowheads in fundus image denote the boundaries of RPE hypopigmentation. Binary and morphometric quantification of RPE degeneration are shown. *P < 0.05; **P < 0.01; ***P < 0.001, Fisher’s exact test for binary; two-tailed t test for morphometry. PM, polymegethism [mean (SEM)]. n = 6–8. (F) Alu RNA fused with S1 RNA aptamer at the 3′ end (Alu-S1) was transfected into mouse embryonic carcinoma cells (mF9) cells. Alu cDNA was detected in the RNase-treated cytoplasmic fraction of these cells, and sequencing using an Alu specific-forward and S1 aptamer-specific reverse primer confirmed the presence of the complementary S1 aptamer sequence in this Alu cDNA.