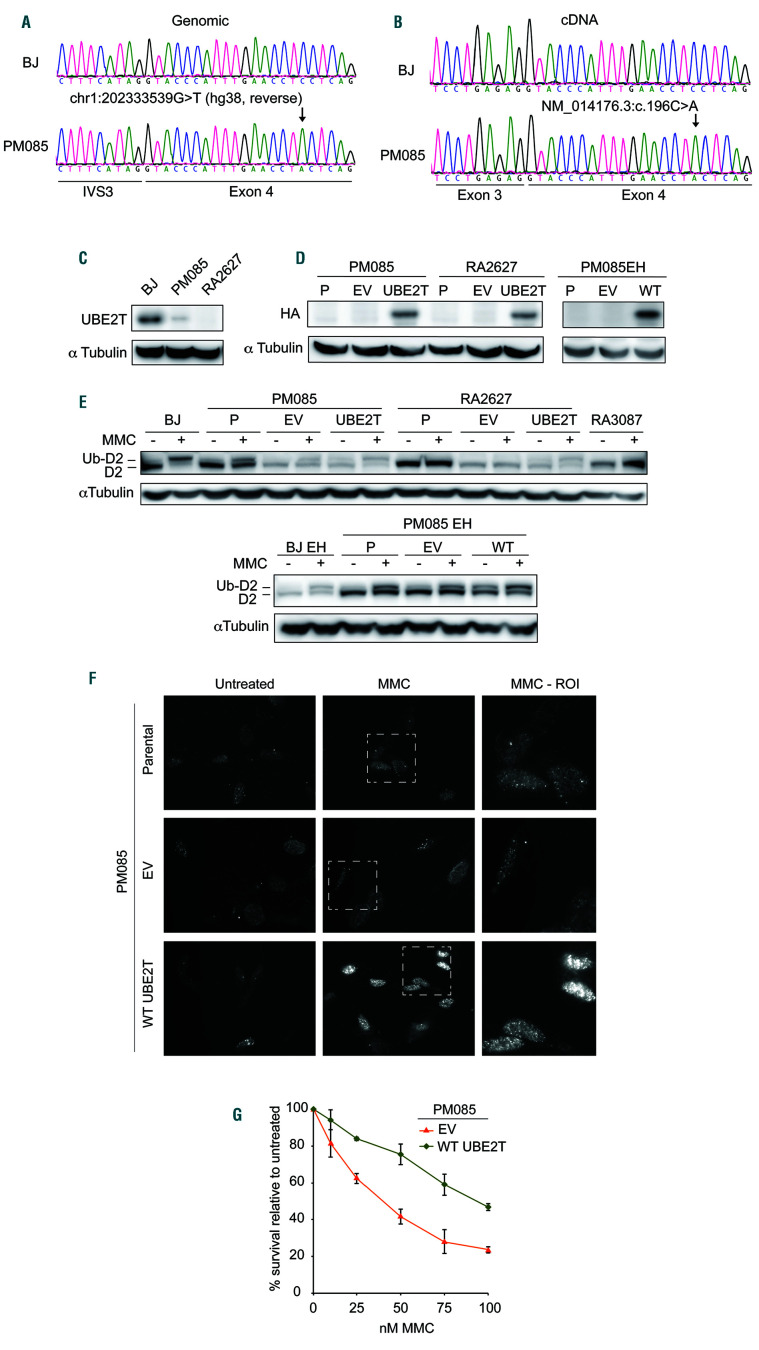
Figure 1.
The proband carries a likely pathogenic UBE2T variant expressed at low levels conferring defective interstrand crosslink repair. (A) Sequencing of genomic DNA extracted from primary fibroblasts (PM085) of the affected individual indicating a homozygous chr1:202333539G>T variant (hg38, reverse). (B) Sequencing of complementary DNA (cDNA) from the proband’s fibroblasts indicating the presence of a variant NM_014176.3:c.196C>A and no evidence of aberrant splicing. Exon numbering reflects ref seq NM_014176.3 since the primers were designed against this transcript.6 (C) Immunoblot with anti-UBE2T antibody in whole cell extract from the proband’s primary fibroblasts (PM085), wildtype BJ fibroblasts (from the American Type Culture Collection) and fibroblasts from an UBE2T/FANCT-null Fanconi anemia patient (RA2627).6 (D) Immunoblot with anti-HA antibody in PM085 (proband) and RA2627 (UBE2T-/-) primary fibroblasts and PM085 EH (immortalized fibroblasts) expressing C-HAFLAG empty vector (EV) or wild-type (WT) UBE2T. HA expression in parental (P) (nontransduced) cells and cells expressing EV or WT UBE2T. (E) Immunoblot with anti-FANCD2 antibody on whole cell extracts of cells treated or not with mitomycin C (MMC). Ub-D2 indicates the monoubiquitinated band. (F) Formation of foci of FANCD2 after MMC treatment in patient-derived PM085 cells (nontransduced parental cells) or cells expressing EV, or WT UBE2T. (G) Cell survival of the proband’s PM085 fibroblasts expressing EV or WT UBE2T after treatment with MMC.