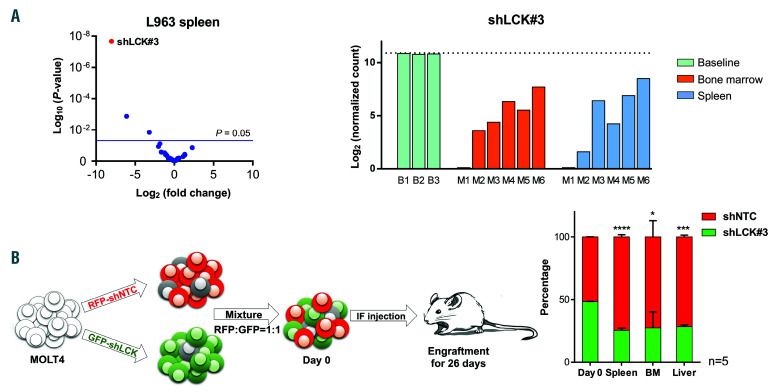
Figure 3.
Loss of lymphocyte cell-specific kinase (LCK) negatively effects propagation potential of the T-cell acute lymphoblastic leukemia (T-ALL) cell line MOLT4 and patient-derived xenograft (PDX) L963 in vivo. (A) Volcano plot derived from the functional in vivo screen representing the magnitude of the fold change (log2) in shRNA abundance derived from leukemia cells isolated from the spleen of PDX L963 on the x-axis. Each dot represents an individual shRNA construct. The y-axis represents the significance in enrichment or depletion of shRNA constructs (log10 scale). Three dots (shLCK#3, shRPL9#1 and shCD19#2) above the blue line are significantly depleted (P<0.05). Bar plot of the normalized shLCK#3 sequencing reads (log2) in leukemic cells derived from the bone marrow (orange) or spleen (blue) of six individual mice (M1-6), relative to the frequency of these reads before transplantation (green, base line B1-3). (B) Schematic representation of the in vivo competitive outgrowth assay. MOLT4 cells were lentivirally transduced with shNTC (red fluorescent protein, RFP) or shLCK#3 (GFP) and intrafemorally injected into five NSG mice in a 1:1 ratio. Mice were culled once symptomatic and the ratio of RFP : GFP positive human leukemic cells in spleen (n=5), bone marrow (n=3) or liver (n=3) determined by flow cytometry. In all mice, the MOLT4 cells carrying shLCK#3 were outcompeted by shNTC cells during engraftment in spleen, marrow and liver. Student's t-test: *P<0.05, ***P<0.005, ****P<0.001.