Figure 2.
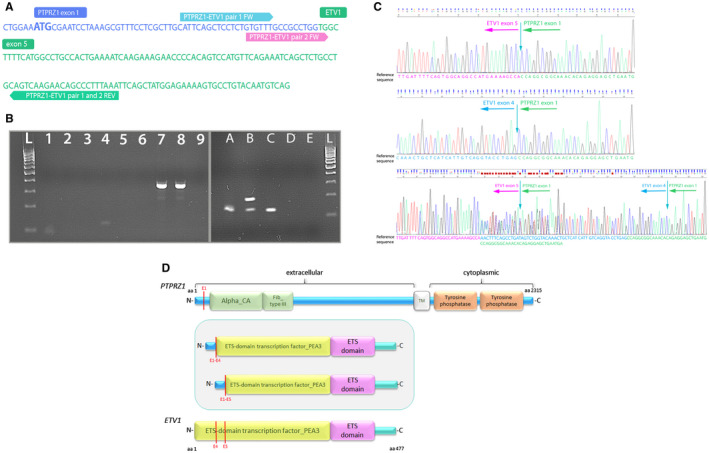
RT‐PCR validation of novel PTPRZ1‐ETV1 fusion. A. Nucleotide sequence of fused PTPRZ1 exon 1 and ETV1 exon 5 with primers designed for RT‐PCR and direct sequencing. B. RT‐PCR for validation of PTPRZ1‐ETV1 fusion present (lane L—DNA 100bp ladder; lanes 1‐3—PCR reactions with primer pair 1 (fusion‐positive sample (88 bp), fusion‐negative sample and no template control (NTC)); lanes 4–6—PCR reactions with primer pair 2 (fusion‐positive sample (121 bp), fusion‐negative sample and no template control (NTC); lanes A–D—PCR validation with primer pair 2, lane E—NTC). Sample B shows two amplified fragments and Sanger sequencing showed it harbors both fusion transcripts, P1E4 (175 bp long fragment) and P1E5 (121 bp long fragment). Sample D showed no amplified product. C. Chromatogram of the RT‐PCR amplified fusion PTPRZ1(exon1)‐ETV1(exon5), PTPRZ1(exon1)‐ETV1(exon4) and both fusion transcripts present (for Sanger sequencing, we used the reverse primer of pair 2). D. Schematic depiction of predicted fusion protein (in the frame) involving PTPRZ1 and ETV1 genes. Red lines represent exons of the PTPRZ1 and ETV1 gene, and also the point of break and fusion. aa = amino acid; Alpha_CA = alpha carbonic anhydrase; Fib_Type III = fibronectin type‐III; TM = transmembrane domain.
