Abstract
Background
Doublets plus anti‐epidermal growth factor receptors (EGFRs) are the preferred upfront option for patients with left‐sided RAS/BRAF wild‐type metastatic colorectal cancer (mCRC). Initial therapy with FOLFOXIRI‐bevacizumab is superior to doublets plus bevacizumab independently from primary tumor sidedness and RAS/BRAF status. No randomized comparison between FOLFOXIRI‐bevacizumab versus doublets plus anti‐EGFRs is available in left‐sided RAS/BRAF wild‐type mCRC.
Materials and Methods
We selected patients with left‐sided RAS and BRAF wild‐type mCRC treated with first‐line FOLFOX‐panitumumab or FOLFOXIRI‐bevacizumab in five randomized trials: Valentino, TRIBE, TRIBE2, STEAM, and CHARTA. A propensity score‐based analysis was performed to compare FOLFOXIRI‐bevacizumab with FOLFOX‐panitumumab.
Results
A total of 185 patients received FOLFOX‐panitumumab and 132 received FOLFOXIRI‐bevacizumab. Median progression‐free survival (PFS) and median overall survival (OS) were 13.3 and 33.1 months in the FOLFOXIRI‐bevacizumab group compared with 11.4 and 30.3 months in the FOLFOX‐panitumumab group (propensity score‐adjusted hazard ratio (HR) for PFS, 0.82; 95% confidence interval (CI), 0.64–1.04; p = .11; propensity score‐adjusted HR for OS, 0.80; 95% CI, 0.59–1.08; p = .14). No significant differences in overall response rate and disease control rate were observed. A statistically nonsignificant difference in favor of FOLFOXIRI‐bevacizumab was observed for OS after secondary resection of metastases. Chemotherapy‐related adverse events were more frequent in the FOLFOXIRI‐bevacizumab group, with specific regard to grade 3 and 4 neutropenia (48% vs. 26%, adjusted p = .001).
Conclusion
Although randomized comparison is lacking, both FOLFOXIRI‐bevacizumab and FOLFOX‐panitumumab are valuable treatment options in left‐sided RAS/BRAF wild‐type mCRC.
Implications for Practice
A propensity score‐based analysis of five trials was performed to compare FOLFOX‐panitumumab versus FOLFOXIRI‐bevacizumab in left‐sided RAS/BRAF wild‐type metastatic colorectal cancer (mCRC). No significant differences were observed, but FOLFOXIRI‐bevacizumab achieved numerically superior survival outcomes versus FOLFOX‐panitumumab. Chemotherapy‐related adverse events were more frequent in the FOLFOXIRI‐bevacizumab group. These observations suggest that although doublet chemotherapy plus anti‐EGFRs remains the preferred treatment in patients with left‐sided RAS/BRAF wild‐type mCRC, FOLFOXIRI‐bevacizumab is a valuable option able to provide similar, if not better, outcomes at the price of a moderate increase in toxicity and may be adopted based on patients’ preference and potential impact on quality of life.
Keywords: Metastatic colorectal cancer, Combination chemotherapy, Bevacizumab, Panitumumab, FOLFOX, FOLFOXIRI
Short abstract
This article reports a propensity score‐based analysis of data from five randomized clinical trials, comparing the efficacy and safety of FOLFOXIRI‐bevacizumab versus FOLFOX‐panitumumab in the subgroup of patients with left‐sided RAS and BRAF wild‐type metastatic colorectal cancer.
Introduction
The combination of a chemotherapy doublet (FOLFOX or FOLFIRI) with an anti‐epidermal growth factor receptor (EGFR) monoclonal antibody is indicated by main guidelines as the preferable initial therapy for patients with unresectable left‐sided RAS and BRAF wild‐type metastatic colorectal cancer (mCRC) [1]. This recommendation is based on the results of the post hoc subgroup analyses of several randomized clinical trials (RCTs) showing a clear benefit from doublets plus anti‐EGFR when compared with doublets with or without bevacizumab in patients with RAS wild‐type tumors originating in the left side of the colon [2, 3]. These findings are also corroborated by molecular data showing a higher prevalence of features of EGFR dependence and a lower occurrence of mechanisms of intrinsic resistance to EGFR inhibition in the left side as compared with the right side [4, 5, 6]. Another upfront option for clinically selected patients with mCRC is the combination of the triplet FOLFOXIRI with bevacizumab that demonstrated improved outcome compared with doublets plus bevacizumab in several RCTs [7, 8, 9, 10]. In clinical practice, triplet chemotherapy is mostly offered to fit patients with poor prognosis and more limited therapeutic options, in particular those with right‐sided primary tumors and/or RAS or BRAF mutated tumors. This is consistent with the under‐representation of patients with left‐sided RAS and BRAF wild‐type tumors in clinical trials investigating the efficacy of the intensification of the upfront chemotherapy backbone in combination with bevacizumab. However, subgroup analyses of available RCTs suggest that patients with left‐sided RAS and BRAF wild‐type tumors may derive benefit from an intensified chemotherapy [8, 11, 12], and the inhibition of angiogenesis is demonstrated as an equally effective strategy in both right‐ and left‐sided tumors, independently of RAS and BRAF mutational status [13, 14].
Whereas the superiority of both doublets plus anti‐EGFR and FOLFOXIRI‐bevacizumab was demonstrated versus doublets plus bevacizumab, nowadays, no evidence from clinical trials comparing these options is available and no studies addressing this question are currently ongoing. Drawing from these considerations, we performed a propensity score‐based analysis including data from five RCTs with the aim of comparing the efficacy and safety of FOLFOXIRI‐bevacizumab versus FOLFOX‐panitumumab in the subgroup of patients with left‐sided RAS and BRAF wild‐type mCRC.
Materials and Methods
Study Population
For the present analysis, we selected patients with RAS and BRAF wild‐type tumors originating from the splenic flexure, descending colon, sigma, and rectum, treated with first‐line FOLFOXIRI‐bevacizumab or FOLFOX‐panitumumab in 5 RCTs: Valentino, TRIBE, TRIBE2, CHARTA, STEAM.
Valentino (NCT02476045) was a multicenter, randomized, open‐label phase II trial that enrolled 229 patients and showed that, in patients with RAS wild‐type mCRC, FOLFOX‐4 plus panitumumab followed by maintenance with single‐agent panitumumab (arm B) achieved inferior progression‐free survival (PFS) compared with the same induction regimen followed by panitumumab plus 5‐fluorouracil/leucovorin (arm A) [15]. TRIBE (NCT00719797) was a multicenter, randomized, open‐label phase III trial that enrolled 508 patients and showed that first‐line FOLFOXIRI‐bevacizumab achieved superior PFS and overall survival (OS) compared with FOLFIRI‐bevacizumab in patients with molecularly unselected mCRC [7]. TRIBE2 (NCT02339116) was a randomized, phase III trial that enrolled 679 patients and showed that upfront FOLFOXIRI‐bevacizumab followed by the reintroduction of the same regimen after progression provided a statistically significant and clinically relevant benefit when compared with the sequential administration of FOLFOX‐bevacizumab and FOLFIRI‐bevacizumab in patients with unresectable mCRC [8]. CHARTA (NCT01321957) was a phase II trial that randomized 250 patients and showed that FOLFOXIRI‐bevacizumab was superior to FOLFOX‐bevacizumab in terms of PFS in the first‐line setting [9]. STEAM (NCT01765582) was a phase II, randomized trial that enrolled 280 patients and showed that first‐line concurrent FOLFOXIRI‐bevacizumab and sequential FOLFOXIRI‐bevacizumab were well tolerated regimens with numerically improved objective response rate, PFS, and liver resection rates compared with FOLFOX‐bevacizumab [10]. The trials included in the present study were conducted in accordance with the Declaration of Helsinki for experiments involving humans, and all patients had given informed consent for trial participation. The analysis was designed in 2020, and a study database was set up to include the information extrapolated by the five trials raw data sets. The members of all trial management committees gave their approval according to a formal protocol.
Statistical Analysis
To deal with systematic differences in the distribution of baseline characteristics between patients treated with FOLFOXIRI‐bevacizumab and those treated with FOLFOX‐panitumumab, a propensity score‐based approach was used with the aim of minimizing the effects of confounding factors and improve the estimation accuracy of treatment effect [16]. The variables considered for the estimation of the propensity score were age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), prior adjuvant chemotherapy, primary tumor resection, liver‐limited disease, and number of metastatic sites. Normalized difference [17] and combined baseline difference [18] were used to check single and global covariates imbalance, respectively, between the two treatment groups. A normalized difference of at least 25% was considered to be indicative of an imbalance between the two treatment groups [19]. Propensity score (i.e., the probability of receiving FOLFOXIRI‐bevacizumab conditional on examined baseline characteristics) was calculated by means of a multivariable logistic regression model. A propensity score‐adjusted analysis was used to estimate the effect of the treatment groups on clinical outcomes. As a sensitivity tool, a propensity score matching analysis with 1:1 ratio, and a caliper of 0.1 was used. Subgroup analyses were performed only using the propensity score‐adjusted method because of the loss of precision due to extremely small sample sizes. A graphical approach was used to assess the distribution of the propensity scores before and after matching. Logistic regression was used to assess the probability of experiencing RECIST response, disease control, and adverse events conditional on the treatment regimen in the propensity score‐adjusted analysis. Conditional logistic regression was used in the propensity score matching analysis. Wald test was used to calculated p values in the context of logistic regression analyses. The reverse Kaplan‐Meier method was used for follow‐up time assessment. The Kaplan‐Meier method and Cox proportional hazards regression model were used for survival analysis. Stratification on the matched pairs was used to estimate the treatment effect on survival outcomes in the propensity score matching analysis. Wald test was used to calculate p values in the context of Cox proportional hazards regression models. PFS was defined as the time between randomization to disease progression or death from any cause. OS was defined as the time between randomization and death from any cause. In the absence of events, PFS and OS times were censored at the last date when the patients were known to be alive. Relapse‐free survival (RFS) was defined as the time between the date of secondary resection with curative intent to disease relapse or death from any cause. RFS analysis was limited to patients who underwent secondary resections with curative intent in the Valentino, TRIBE, and TRIBE2 trials, because information about the date of surgery was not available for patients in the CHARTA and STEAM trials. Only patients with measurable disease at the time of randomization and at least one instrumental evaluation of tumor response were included in activity analyses. Threshold for statistical significance was set to p value = .05, and all statistical tests were two‐sided. Statistical analyses were performed using the R software (version 3.5.0).
Results
Patients’ Characteristics
The process of patients’ selection is illustrated in supplemental online Figure 1. A total of 317 patients with left‐sided RAS and BRAF wild‐type mCRC were included in the propensity score‐adjusted analysis: 185 treated with FOLFOX‐panitumumab and 132 with FOLFOXIRI‐bevacizumab. Patients’ characteristics are illustrated in Table 1. We observed a significant global difference between the two treatment groups in the distribution of the variables considered for the estimation of the propensity score (p = .04). Indeed, the normalized difference showed a significant imbalance between the distributions of the two groups for prior adjuvant chemotherapy and primary tumor resection. The baseline distribution of propensity scores is shown in supplemental online Figure 2. For the propensity score‐matched sensitivity analysis, 124 patients in the FOLFOX‐panitumumab group and 124 patients in the FOLFOXIRI‐bevacizumab group were successfully matched. The distribution of propensity scores after matching is shown in supplemental online Figure 2. We did not observe a significant global difference in the distribution of the variables after matching (p = .76).
Table 1.
Patients’ characteristics
| Characteristics | FOLFOX‐P (n = 185), n (%) | FOLFOXIRI‐B (n = 132), n (%) | Standardized difference, % |
|---|---|---|---|
| Age | 24 | ||
| <70 | 143 (77) | 114 (86) | |
| ≥70 | 42 (23) | 18 (14) | |
| Sex | 6 | ||
| Female | 58 (31) | 45 (34) | |
| Male | 127 (69) | 87 (66) | |
| ECOG PS | 4 | ||
| 0 | 137 (74) | 100 (76) | |
| 1 | 48 (26) | 32 (24) | |
| Prior adjuvant chemotherapy | 36 | ||
| No | 155 (84) | 125 (95) | |
| Yes | 30 (16) | 7 (5) | |
| Primary tumor resection | 27 | ||
| No | 71 (38) | 68 (52) | |
| Yes | 114 (62) | 64 (48) | |
| Liver‐limited disease | 2 | ||
| No | 114 (62) | 80 (61) | |
| Yes | 71 (38) | 52 (39) | |
| Number of metastatic sites | 8 | ||
| 1 | 104 (56) | 69 (52) | |
| >1 | 81 (44) | 63 (48) |
Abbreviations: B, bevacizumab; ECOG PS, Eastern Cooperative Oncology Group performance status; P, panitumumab.
Efficacy and Activity
After a median follow‐up time of 39.6 months (interquartile range [IQR], 31.9–45.5), a total of 279 events for PFS and 183 events for OS were observed. The median follow‐up time was 36.5 months (IQR, 31.6–44.4) for patients treated with FOLFOX‐panitumumab and 44.3 months (IQR, 33.3–51.4) for patients treated with FOLFOXIRI‐bevacizumab. In the propensity score‐adjusted analysis, no differences between patients treated with FOLFOXIRI‐bevacizumab and those receiving FOLFOX‐panitumumab were observed for PFS (median PFS, 13.3 vs. 11.4 months respectively; adjusted hazard ratio [HR], 0.82; 95% confidence interval [CI], 0.64–1.04; p = .11; Fig. 1, panel A) and OS (median OS, 33.1 vs. 30.3, respectively; adjusted HR, 0.80; 95% CI, 0.59–1.08; p = .14; Fig. 1, panel B). The results of the propensity score‐matched sensitivity analyses were consistent with the propensity score‐adjusted analyses (supplemental online Fig. 3).
Figure 1.
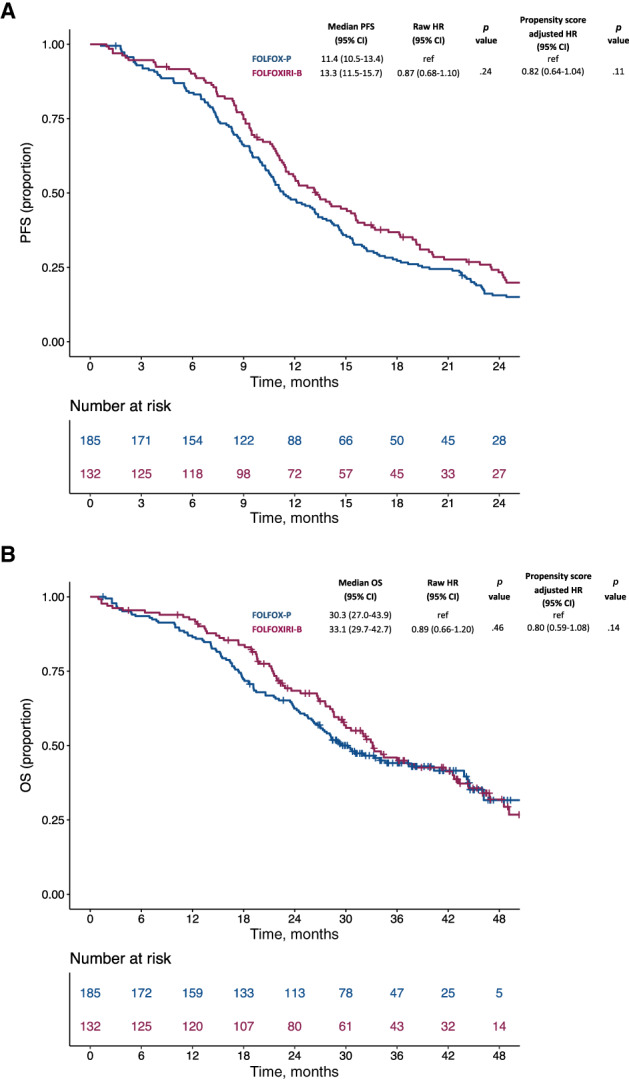
Kaplan‐Meier curves for PFS (A) and OS (B) according to treatment in the propensity score‐adjusted analysis. Blue lines indicate patients treated with FOLFOX‐panitumumab, whereas violet lines indicate patients treated with FOLFOXIRI‐bevacizumab.Abbreviations: B, bevacizumab; CI, confidence interval; HR, hazard ratio; OS, overall survival; P, panitumumab; PFS, progression‐free survival.
No difference was observed in the propensity score‐adjusted analysis in terms of response rate (73% with FOLFOXIRI‐bevacizumab vs. 77% with FOLFOX‐panitumumab; adjusted OR, 0.79; 95% CI, 0.45–1.37; p = .40) or disease control rate (96% with FOLFOXIRI‐bevacizumab vs. 95% with FOLFOX‐panitumumab; adjusted OR, 1.09; 95% CI, 0.34–3.50; p = .89; Table 2). The results of the propensity score‐matched sensitivity analysis were consistent with the propensity score‐adjusted analysis (supplemental online Table 1).
Table 2.
Propensity score‐adjusted analysis for activity according to treatment
| Treatment | Response (CR + PR) | Clinical benefit (CR + PR + SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Raw OR (95% CI) a | p value a | PS‐adjusted OR (95% CI) a | p value a | n (%) | Raw OR (95% CI)* | p value a | PS‐adjusted OR (95% CI)* | p value a | |
| FOLFOX‐P (n = 172) | 133 (77) | ref | .59 | ref | .40 | 164 (95) | ref | .78 | ref | .89 |
| FOLFOXIRI‐B (n = 126) | 92 (73) | 0.86 (0.50–1.47) | 0.79 (0.45–1.37) | 121 (96) | 1.18 (0.38–3.70) | 1.09 (0.34–3.50) | ||||
Logistic regression Wald test.
Abbreviations: B, bevacizumab; CI, confidence interval; CR, complete response; OR, odds ratio; p, panitumumab; PR, partial response; PS, propensity score; ref, reference; SD, disease stabilization.
Secondary Resection of Metastases
Secondary resection rate did not differ between the FOLFOXIRI‐bevacizumab and the FOLFOX‐panitumumab group (22% vs. 18%, respectively; p = .51; supplemental online Table 2). Similar results were observed in the propensity score‐matched sensitivity analysis (supplemental online Table 3). Among patients who underwent radical resection of metastases, no significant difference was observed in terms of postresection RFS (adjusted HR, 0.62; 95% CI, 0.32–1.18; p = .146) and postresection OS (adjusted HR, 0.30; 95% CI, 0.08–1.07; p = .064; supplemental online Fig. 4).
Safety
In the propensity score‐adjusted analysis, FOLFOXIRI‐bevacizumab was associated with a higher incidence of chemotherapy‐related grade 3 or 4 adverse events (59% vs. 46%; adjusted p = .04), in particular neutropenia (48% vs. 26%, adjusted p = .001) but not febrile neutropenia (6% vs. 3%; adjusted p = .24; Table 3). In the propensity score‐matched sensitivity analysis, the higher incidence of grade 3 or 4 neutropenia in the FOLFOXIRI‐bevacizumab group was confirmed (48% vs. 30%; p = .03), but no statistically significant differences in the overall incidence of chemotherapy‐related adverse events was observed (59% vs. 51%; p = .39), probably because of the smaller sample size (supplemental online Table 4).
Table 3.
Propensity score‐adjusted analysis of the incidence of chemotherapy‐related adverse events of grade 3 or 4 according to treatment
| Grade 3/4 adverse events | Total (n = 317), n (%) | FOLFOX‐P (n = 185), n (%) | FOLFOXIRI‐B (n = 132), n (%) | Raw p value a | PS‐adjusted p value a |
|---|---|---|---|---|---|
| All | 163 (51) | 85 (46) | 78 (59) | .02 | .04 |
| Neutropenia | 112 (35) | 49 (26) | 63 (48) | <.001 | .001 |
| Febrile neutropenia | 13 (4) | 5 (3) | 8 (6) | .15 | .24 |
| Anemia | 9 (3) | 4 (2) | 5 (4) | .40 | .52 |
| Thrombocytopenia | 8 (3) | 5 (3) | 3 (2) | .81 | .77 |
| Nausea | 11 (3) | 5 (3) | 6 (5) | .38 | .38 |
| Vomiting | 7 (2) | 2 (1) | 5 (4) | .13 | .13 |
| Stomatitis/oral mucositis | 22 (7) | 14 (8) | 8 (6) | .60 | .67 |
| Diarrhea | 42 (13) | 25 (14) | 17 (13) | .87 | .82 |
| Peripheral neuropathy | 13 (4) | 8 (4) | 5 (4) | .81 | .68 |
Logistic regression Wald test.
Abbreviations: B, bevacizumab; P, panitumumab; PS, propensity score.
Discussion
To compare the efficacy, activity, and safety of FOLFOXIRI‐bevacizumab versus FOLFOX‐panitumumab in patients with unresectable left‐sided RAS and BRAF wild‐type mCRC, we performed a propensity score‐based analysis of five RCTs. No statistically significant differences were reported in terms of activity or efficacy, but FOLFOXIRI‐bevacizumab was associated with not statistically significant improvements in PFS and OS, as well as RFS and OS after secondary resection with curative intent, at the price of a higher rate of grade 3 or 4 chemotherapy‐related adverse events, particularly in terms of neutropenia.
We acknowledge that the two limitations of our analysis are the retrospective nature without a formal a priori hypothesis and the lack of randomization. Even if patients in the two treatment groups were enrolled in a relatively recent time frame, some unbalances in baseline prognostic features were due to differences in studies’ inclusion criteria in terms of age, ECOG PS, and prior adjuvant therapy. In particular, no upper age limit was set in the Valentino and CHARTA studies, whereas in the TRIBE, TRIBE2, and STEAM trials, only patients aged <75 years were eligible and those >70 years only if their ECOG PS was 0. Nevertheless, propensity score adjustment was applied to take into account such differences, thus limiting the bias related to a nonrandomized comparison. Even if all trials investigating FOLFOXIRI‐bevacizumab adopted a maintenance therapy with 5‐fluoruracil/leucovorin plus bevacizumab until disease progression, unacceptable toxicity, or consent withdrawal, the Valentino study investigated two different maintenance strategies: 5‐fluoruracil/leucovorin plus panitumumab or panitumumab alone. The trial showed that the anti‐EGFR monotherapy was inferior compared with the combination of the anti‐EGFR and 5‐fluoruracil/leucovorin in terms of PFS, primary endpoint of the study, with no OS difference. Therefore, in this analysis, around 50% of patients in the FOLFOX‐panitumumab group likely received a suboptimal maintenance therapy, and this may have partially affected the results, at least in terms of PFS. Also, the differences across trials in the planned duration of the induction therapy (4 months in the Valentino study versus variable durations from 4 to 6 months in the trials investigating FOLFOXIRI‐bevacizumab) may have somehow affected our results.
Our clinical findings are supported by some biological considerations. Indeed, the selection of left‐sided RAS and BRAF wild‐type tumors responsive to EGFR inhibition may be refined by the concomitant assessment of uncommon molecular alterations associated with primary resistance such as HER2 amplification, PI3KCA/PTEN mutations, subclonal RAS mutations, gene fusions, and microsatellite instability (PRESSING panel) [4]. In the subgroup of patients with left‐sided RAS and BRAF wild type, PRESSING‐negative mCRC enrolled in the Valentino study, the negative molecular hyperselection allowed to achieve unprecedented survival outcomes following first‐line FOLFOX‐panitumumab. Therefore, we acknowledge that comparing FOLFOXIRI‐bevacizumab with FOLFOX‐panitumumab in a molecularly hyperselected population might have provided different results. Unfortunately, whereas deep molecular characterization by means of next‐generation sequencing was available in the more recent Valentino and TRIBE2 studies, such extensive molecular information was not available for all the trials included in the present analysis, and a comparison of FOLFOXIRI‐bevacizumab with FOLFOX‐panitumumab in a molecularly hyperselected population was not possible.
Subgroup analyses of RCTs suggest that also left‐sided RAS and BRAF wild‐type tumors may achieve benefit (and probably a higher extent of benefit) from the intensification of the upfront although backbone in combination with the antiangiogenic agent bevacizumab [11, 20, 21]. Therefore, although FOLFOXIRI‐bevacizumab is a valuable option for fit patients with more aggressive and treatment‐resistant cancers, it may achieve its maximal efficacy in the subgroup of patients with better prognosis and responsiveness to available agents, including chemotherapy and antiangiogenics.
Interestingly, in a previous study analyzing the pathological response of liver metastases resected after triplet plus either bevacizumab or cetuximab, we showed a higher extent and frequency of tumor regression and necrosis in the bevacizumab treated group [22]. Given the prognostic relevance of histopathologic response in CRC [23] and the results reported in this analysis in patients undergoing secondary resection of metastases, we speculate that FOLFOXIRI‐bevacizumab may also be offered as a valuable conversion treatment option in patients with initially unresectable, left‐sided RAS and BRAF wild‐type mCRC.
Regarding safety, the expected higher rate of chemotherapy‐related adverse events following FOLFOXIRI‐bevacizumab was confirmed. When looking at specific adverse events, only neutropenia was significantly more frequent with the triplet plus bevacizumab. A previous pooled analysis of TRIBE and TRIBE2 studies showed that elderly patients (i.e., those >70 years old) have a higher risk of experiencing severe toxicity with this intensified regimen [24]. Of note, granulocyte colony‐stimulating factor was not recommended as primary prophylaxis in trials investigating FOLFOXIRI‐bevacizumab, considering the relatively low overall rate of febrile neutropenia (6%) [25]. In contrast, skin rash is obviously the most frequent class‐specific adverse event potentially affecting patients’ tolerance and quality of life during anti‐EGFR–containing regimens [26].
Conclusion
Although acknowledging that the level of evidence of this analysis is not adequate to drive clinical decision‐making, it is rather unlikely that RCTs addressing this issue and requiring high numbers of patients will be designed and conducted in the future. In properly selected patients, both FOLFOXIRI‐bevacizumab and FOLFOX/FOLFIRI plus anti‐EGFRs are highly active upfront regimens able to significantly increase patients’ survival thanks to a deep impact on the initial and subsequent disease course, including the conversion to secondary resection of metastases and maximal shrinkage of initial tumor burden. Although doublet chemotherapy plus anti‐EGFRs remains the preferred option in patients with left‐sided RAS and BRAF wild‐type mCRC, FOLFOXIRI‐bevacizumab is a valuable option able to provide similar outcome results at the price of a moderate increase in toxicity, and may be adopted based on patients’ preference, attitudes, expectations and compliance, potential impact on quality of life, and psychosocial context. Present results underline the potential added value of the intensified upfront chemotherapy backbone in this subgroup of patients, thus supporting the rationale for randomized trials currently ongoing that aim at comparing triplets plus anti‐EGFRs and doublets plus anti‐EGFRs as first‐line therapy for patients with unresectable mCRC [27, 28].
Author Contributions
Conception/design: Filippo Pietrantonio, Giovanni Fucà, Chiara Cremolini
Provision of study material or patients: Filippo Pietrantonio, Hans‐Joachim Schmoll, Johanna C. Bendell, Federica Morano, Salvatore Corallo, Monica Niger, Sara Lonardi, Filippo de Braud, Maria Di Bartolomeo, Alfredo Falcone, Chiara Cremolini
Collection and/or assembly of data: Giovanni Fucà, Daniele Rossini, Carlotta Antoniotti, Beatrice Borelli, Alessandra Raimondi, Federica Marmorino, Alessandra Boccaccino, Gianluca Masi
Data analysis and interpretation: Filippo Pietrantonio, Giovanni Fucà, Luca Boni, Chiara Cremolini
Manuscript writing: Filippo Pietrantonio, Giovanni Fucà, Luca Boni, Chiara Cremolini
Final approval of manuscript: Filippo Pietrantonio, Giovanni Fucà, Daniele Rossini, Hans‐Joachim Schmoll, Johanna C. Bendell, Federica Morano, Carlotta Antoniotti, Salvatore Corallo, Beatrice Borelli, Alessandra Raimondi, Federica Marmorino, Monica Niger, Alessandra Boccaccino, Gianluca Masi, Sara Lonardi, Luca Boni, Filippo de Braud, Maria Di Bartolomeo, Alfredo Falcone, Chiara Cremolini
Disclosures
Daniele Rossini: Takeda (H); Hans‐Joachim Schmoll: Roche (SAB‐Advisory role); Johanna C. Bendell: Gilead, Roche, Bristol‐Myers Squibb, Five Prime, Lilly, Merck, MedImmune, Celgene, Taiho, Macrogenics, GlaxoSmithKline, Novartis, OncoMed, LEAP, TG Therapeutics, AstraZenca, BI, Daiichi Sankyo, Bayer, Incyte, Apexigen, Array, Sanofi, ARMO, Ispen, Merrimack, Oncogenex, FORMA, Arch Oncology, Prelude Oncology, Pfizer, Evelo, Innate Pharma, Cyteir, Bicycle, Relay Therapeutics, Amgen, Seattle Genetics, Beigene, Agios (C/A [Institution], RF [Institution]), EMD Serono, Koltan, SynDevRex, Forty Seven, AbbVie, Onyx, Takeda, Eisai, Celldex, Cytomx, Nektar, Boston Biomedical, Tarveda, Tyrogenex, Marshall Edwards, Pieris, Mersana, Calithera, Blueprint, Merus, Jacobio, Effector, Novocare, Arrys,Tracon,Sierra,Unum Therapeutics, Vyriad, Harpoon, ADC, Millennium, ImClone, Acerta Pharma, Rgenix, Bellicum, Gossamer Bio, Arcus Bio, Tempest Tx, Shatttuck Labs, Synthorx, Revolution Medicines, Zymeworks, Scholar Rock, NGM Biopharma, Stemcentrx, CALGB, Foundation Bio, Morphotex, Ongologie, NuMab, AtlasMedx (RF [Institution]), Phoenix Bio, Molecular Bio, Tizona, Janssen, Tolero, Translational Drug Development, Moderna Therapeutics, Tanabe Research Labs, Continuum Clinical, Piper Biotech, Samsung Bioepios (C/A [Institution]); Federica Morano: Servier (H); Sara Lonardi: Amgen, Merck Serono, Eli Lilly & Co (C/A), Roche, Eli Lilly & Co, Bristol‐Myers Squibb, Servier, Merck Serono (Other‐Speaker's bureau), Amgen, Merck Serono (RF); Filippo de Braud: Amgen, Roche, Novartis (C/A); Alfredo Falcone: Bayer, Bristol, Eli Lilly & Co, Merck, Pierre‐Fabre, Roche, Servier (C/A, H [Personal]), Astra‐Zeneca, Bayer, Bristol, Eli Lilly & Co, Merck, Merck Sharpe & Dohme, Novartis, Roche, Sanofi, Servier (Other‐financial support for clinical trials [Institutional]), Fondazione GONO (Italy), Fondazione ARCO (Italy) (Leadership role in research groups/foundations); Chiara Cremolini: Roche, Amgen, Inc, Bayer AG, and Servier Laboratories (H, SAB), Merck‐Serono (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Supplemental Tables
Disclosures of potential conflicts of interest may be found at the end of this article.
Editor's Note: See the related article, “FOLFOXIRI plus Bevacizumab Versus FOLFOX plus Panitumumab for Metastatic Left‐Sided RAS/BRAF Wild‐Type Colorectal Cancer: Which “Side” Are You On?” by Irene S. Yu and Jonathan M. Loree, on page 277 of this issue.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Yoshino T, Arnold D, Taniguchi H et al. Pan‐Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO‐ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 2018;29:44–70. [DOI] [PubMed] [Google Scholar]
- 2. Holch JW, Ricard I, Stintzing S et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta‐analysis of first‐line clinical trials. Eur J Cancer 2017;70:87–98. [DOI] [PubMed] [Google Scholar]
- 3. Arnold D, Lueza B, Douillard JY et al. Prognostic and predictive value of primary tumour side in patients with RAS wild‐type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017;28:1713–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cremolini C, Morano F, Moretto R et al. Negative hyper‐selection of metastatic colorectal cancer patients for anti‐EGFR monoclonal antibodies: The PRESSING case‐control study. Ann Oncol 2017;28:3009–3014. [DOI] [PubMed] [Google Scholar]
- 5. Morano F, Corallo S, Lonardi S et al. Negative hyperselection of patients with RAS and BRAF wild‐type metastatic colorectal cancer who received panitumumab‐based maintenance therapy. J Clin Oncol 2019;37:3099–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Missiaglia E, Jacobs B, D'Ario G et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014;25:1995–2001. [DOI] [PubMed] [Google Scholar]
- 7. Loupakis F, Cremolini C, Masi G et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609–1618. [DOI] [PubMed] [Google Scholar]
- 8. Cremolini C, Antoniotti C, Rossini D et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): A multicentre, open‐label, phase 3, randomised, controlled trial. Lancet Oncol 2020;21:497–507. [DOI] [PubMed] [Google Scholar]
- 9. Schmoll HJ, Garlipp B, Junghanss C et al. CHARTA: FOLFOX+bevacizumab +/‐ irinotecan in advanced colorectal cancer (CRC)—Final results of the randomized phase II trial of the AIO (KRK 0209). J Clin Oncol 2017;35:658a. [Google Scholar]
- 10. Hurwitz HI, Tan BR, Reeves JA et al. Phase II randomized trial of sequential or concurrent FOLFOXIRI‐bevacizumab versus FOLFOX‐bevacizumab for metastatic colorectal cancer (STEAM). The Oncologist 2019;24:921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cremolini C, Loupakis F, Antoniotti C et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first‐line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open‐label, phase 3 TRIBE study. Lancet Oncol 2015;16:1306–1315. [DOI] [PubMed] [Google Scholar]
- 12. Cremolini C, Antoniotti C, Lonardi S et al. Primary tumor sidedness and benefit from FOLFOXIRI plus bevacizumab as initial therapy for metastatic colorectal cancer. Retrospective analysis of the TRIBE trial by GONO. Ann Oncol 2018;29:1528–1534. [DOI] [PubMed] [Google Scholar]
- 13. Loupakis F, Yang D, Yau L et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 2015;107:dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loupakis F, Hurwitz HI, Saltz L et al. Impact of primary tumour location on efficacy of bevacizumab plus chemotherapy in metastatic colorectal cancer. Br J Cancer 2018;119:1451–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pietrantonio F, Morano F, Corallo S et al. Maintenance therapy with panitumumab alone vs panitumumab plus fluorouracil‐leucovorin in patients with RAS wild‐type metastatic colorectal cancer: A phase 2 randomized clinical trial. JAMA Oncol 2019;5:1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC. The use of propensity score methods with survival or time‐to‐event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33:1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Imbens GW, Wooldridge JM. Recent developments in the econometrics of program evaluation. J Econ Lit 2009;47:5–86. [Google Scholar]
- 18. Hansen BB, Bowers J. Covariate balance in simple, stratified and clustered comparative studies. Statist Sci 2008;23:219–236. [Google Scholar]
- 19. Stuart EA, Rubin DB. Best practices in quasi–experimental designs: Matching methods for causal inference. In Osborne J, ed. Best Practices in Quantitative Methods. Thousand Oaks, CA: Sage; 2008;155–176. [Google Scholar]
- 20. Modest DP, Fischer von Weikersthal L, Decker T et al. Sequential versus combination therapy of metastatic colorectal cancer using fluoropyrimidines, irinotecan, and bevacizumab: A randomized, controlled study‐XELAVIRI (AIO KRK0110). J Clin Oncol 2019;37:22–32. [DOI] [PubMed] [Google Scholar]
- 21. Goey KKH, Elias SG, van Tinteren H et al. Maintenance treatment with capecitabine and bevacizumab versus observation in metastatic colorectal cancer: Updated results and molecular subgroup analyses of the phase 3 CAIRO3 study. Ann Oncol 2017;28:2128–2134. [DOI] [PubMed] [Google Scholar]
- 22. Cremolini C, Milione M, Marmorino F et al. Differential histopathologic parameters in colorectal cancer liver metastases resected after triplets plus bevacizumab or cetuximab: A pooled analysis of five prospective trials. Br J Cancer 2018;118:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cremolini C, Casagrande M, Loupakis F et al. Efficacy of FOLFOXIRI plus bevacizumab in liver‐limited metastatic colorectal cancer: A pooled analysis of clinical studies by Gruppo Oncologico del Nord Ovest. Eur J Cancer 2017;73:74–84. [DOI] [PubMed] [Google Scholar]
- 24. Marmorino F, Rossini D, Lonardi S et al. Impact of age and gender on the safety and efficacy of chemotherapy plus bevacizumab in metastatic colorectal cancer: A pooled analysis of TRIBE and TRIBE2 studies. Ann Oncol 2019;30:1969–1977. [DOI] [PubMed] [Google Scholar]
- 25. Cremolini C, Antoniotti C, Stein A et al. Individual patient data meta‐analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol 2020;38:3314–3324. [DOI] [PubMed] [Google Scholar]
- 26. Raimondi A, Di Maio M, Morano F et al. Health‐related quality of life in RAS wild‐type metastatic colorectal cancer patients treated with panitumumab‐based first‐line treatment strategy: A pre‐specified secondary analysis of the Valentino study. Eur J Cancer 2020;135:230–239. [DOI] [PubMed] [Google Scholar]
- 27. Borelli B, Moretto R, Lonardi S et al. TRIPLETE: A randomised phase III study of modified FOLFOXIRI plus panitumumab versus mFOLFOX6 plus panitumumab as initial therapy for patients with unresectable RAS and BRAF wild‐type metastatic colorectal cancer. ESMO Open 2018;3:e000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazard T, Ghiringhelli F, Mollevi C et al. UCGI 28 Panirinox: A randomized phase II study assessing Panitumumab + FOLFIRINOX or mFOLFOX6 in RAS and BRAF wild type metastatic colorectal cancer patients (mCRC) selected from circulating DNA analysis. Ann Oncol 2018;29(suppl 8):viii150–viii204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Supplemental Tables


