Abstract
Bridged bicyclic ketals display a range of bioactivities. Their catalytic enantioselective synthesis from achiral, acyclic 1,1-disubstituted alkene diols is disclosed. This reaction combines asymmetric catalysis with a distal radical migration. Alkynes and arenes undergo the group transfer. Product distribution depends on substrate and conditions and informs mechanistic analysis.
Graphical Abstract

Bicyclic ketals via copper-catalyzed enantioselective bis(cyclization) involving radical group transfer is disclosed.
Bridged bicyclic ketals (e.g. those in Figure 1) are rigid organic structures composed of saturated cyclic ethers that can place substituents in precise relative locations in three dimensions.1 Bridged bicyclic ketal natural products include the zaragozic acids, a class of squalene synthesis inhibitors,2 and the saliniketals, which have demonstrated inhibition of ornithine decarboxylase induction.3 A bridged bicyclic ketal that inhibits sodium-dependent glucose cotransporter 2 has been developed to treat type 2 diabetes (e.g. Steglatro).4 An alkyne-substituted bridged bicyclic ketal has demonstrated cytotoxicity toward leukemia cells.5
Figure 1.

Bioactive bridged bicyclic ketals
Microbial production is a common route to complex bridged bicyclic ketals.6–7 Considerable effort has also been put toward their de novo chemical synthesis.1 A ketal stereocenter is frequently created via cyclization of two pendant alcohols onto a ketone, where the lowest energy diastereomer is typically favored.1 The acid-catalyzed addition of an alcohol to an enol ether is a related approach.8 Transition metal-catalyzed [including (Pt),9 (Ag)10 and (Au)11] additions of alcohols, carbonyls or carbenes [catalyzed by (Rh)12] to alkynes have also been applied.1
In recent years, excellent progress has also been made toward the catalytic enantioselective synthesis of spirocyclic ketals.13–16 The catalytic enantioselective synthesis of bridged bicyclic ketals is less common, and all such methods reported to date involve catalytic enantioselective conjugate addition of various nucleophiles to enones, followed by diastereoselective addition of pendant alcohols to the resident carbonyl (Scheme 1a). Along these lines, Shi and co-workers have reported a [Pd]-catalyzed method.17 Liu and co-workers have reported a Bronsted acid catalyzed method.18 Related organocatalytic methods have been reported independently by Pan,19 Jorgensen,20 and Franzen.21 A copper-catalyzed cyclization of a ketone onto an alkene in the presence of DMSO for the synthesis of (±)-benzobicyclo[3.2.1]octanes has also been disclosed.22
Scheme 1.
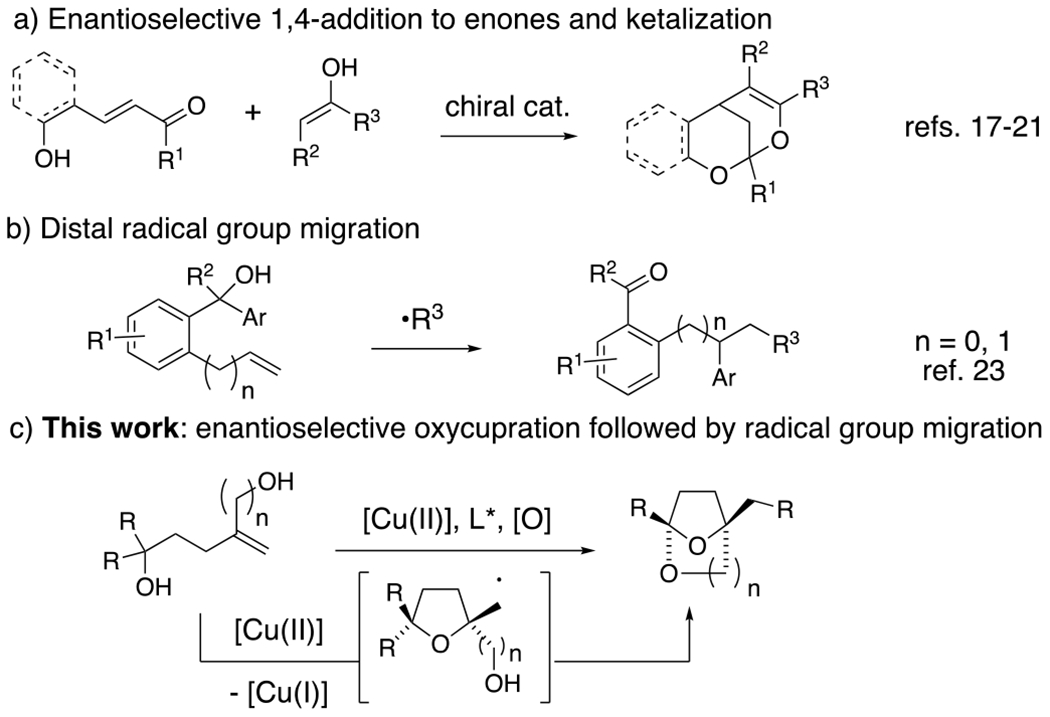
Bridged bicyclic ketals and distal group migration
We report herein a strategy for the enantioselective synthesis of chiral bridged bicyclic spiroketals that involves a radical group transfer step (Scheme 1c). Radical migration reactions enable the scission and transfer of unsaturated carbon moieties within a molecule. A number of recent reports have highlighted the power of the distal radical migration strategy in organic synthesis (e.g. Scheme 1b).23–26 Herein we report the first distal radical migration reaction to occur in the context of asymmetric catalysis.
In recent years, our group has established that enantioenriched tetrahydrofurans can be synthesized from copper-catalyzed carboetherifications of 4-pentenols.27–30 Enantioselective cis-oxycupration across the alkene,26 followed by in situ homolysis of the resulting C-Cu(II) bond is proposed to result in carbon radical formation (Scheme 1c and Scheme 4, vide infra). Subsequent C-C bond formation can occur via addition of the carbon radical to styrenes28–29 or pendant arenes,27, 30 which, in prior reports, underwent subsequent oxidation under the conditions to form higher substituted alkenes or arenes (net C-H functionalization). We have also shown that copper(II)-promoted oxyamination and dioxygenation can occur in the absence of π-bond radical group acceptors,31 where the carbon-heteroatom bond formation is thought to occur via a Cu(III) intermediate and a Kharash-Sosnovsky type mechanism.32–33 Although we anticipated 1,1-diphenyl-4-methylene-1,6-hexanediol (1a) would undergo the oxycupration step, it was unclear if the resulting radical intermediate would favor 1,4-phenyl transfer, followed by cyclization to form bridged bicyclic ketal 2a, or a Cu(III)-facilitated dioxygenation to form bis(ether) 3a (Table 1). In the event, we found that the reaction conditions and the substrate structure both impact the course of the reaction and subsequently, product formation (vide infra).
Scheme 4.
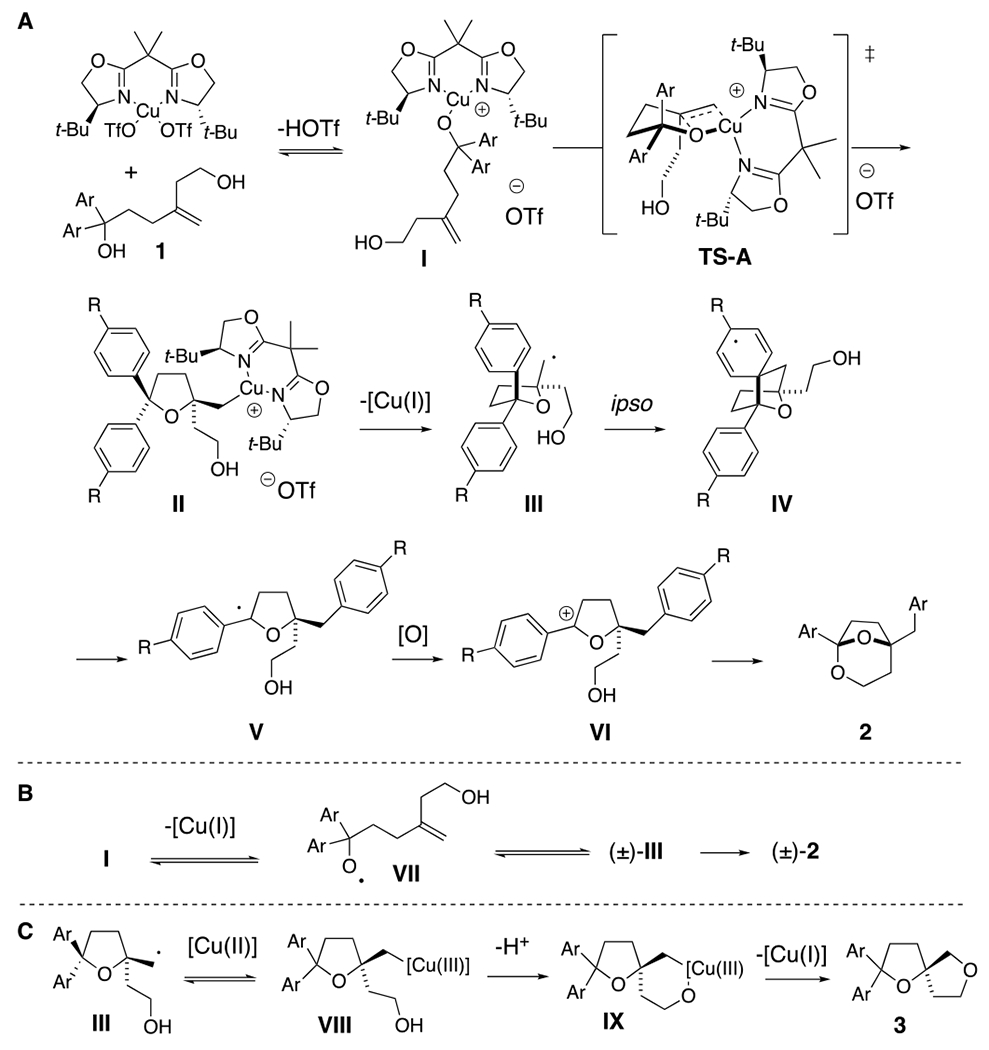
Proposed mechanism to 2 and 3
Table 1.
Effect of Reaction Conditionsa
 | ||||
|---|---|---|---|---|
| entry | change from standard conditions | 2a + 3a (%) | 2a:3ab | ee (%) 2ac |
| 1 | None | 64 | >10:1 | 92 |
| 2 | L1, 48 h | 61 | 5:1 | -- |
| 3 | L3 | 68 | 4:1 | 57 |
| 4 | 24 h | 55 | >10:1 | 92 |
| 5 | 15 mol % [Cu], 48 h | 46 | >20:1 | nd |
| 6 | 25 mol % [Cu], 72 h | 72 | 5:1 | 92 (3a: 86) |
| 7 | 30 mol % [Cu], 48 h | 62 | 3:1 | nd |
| 8 | 60 mol % [Cu], 48 h | 70 | 1.7:1 | nd |
| 9d | 50 mol % [Cu], L1, 48 h | ca. 90 | 2:1 | nd |
| 10 | Ag2CO3 (1.5 equiv) instead of MnO2 | 76 | 5:1 | nd |
| 11 | 25 mol % [Cu], 10 % KMnO4 combined with the MnO2 | 68 | 1.4:1 | nd |
| 12 | 25 mol % [Cu], PhCH3 instead of PhCF3, 48 h | 51 | 4:1 | 78 |
| 13 | DCE instead of PhCF3, 48 h | 38 | >10:1 | 50 |
| 14d | Cu(NTf2)2 instead of Cu(OTf)2, 48 h | 53 | 1.5:1 | nd |
Reactions run on 0.1 mmol of 1a in 1 mL of PhCF3 with a [Cu]:L ratio of 1:1.25 in a sealed tube (heated in 120 °C oil bath). Isolated yield following chromatography on silica gel, unless otherwise noted.
Ratio obtained from analysis of the crude 1H NMR.
Enantiomeric excess measured by chiral HPLC.
Estimated crude 1H NMR yield. Nd = not determined.
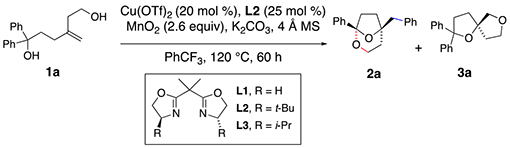
Various reaction conditions for the oxidative transformation of 1a are presented in Table 1. Bridged bicyclic ketal 2a is formed in 64% yield, >10:1 selectivity of 2a over 3a, in 92% ee, using 20 mol% Cu(OTf)2 and 25 mol% of (S,S)-t-BuBox (L2) as catalyst, MnO2 (2.6 equiv) as oxidant, with K2CO3 and 4 Å mol. sieves as additives, in PhCF3 at 120 °C for 60 h (Table 1, entry 1). The first indication of the major product’s identity as a ketal was its distinctive 13C NMR signal at 106 ppm.
A ligand effect on product ratio was observed: when the reaction was run using the achiral bis(oxazoline) ligand (L1), a 5:1 ratio of racemic ketal 2a and bis(ether) 3a was obtained in a combined 61% yield (entry 2). When (S,S)-i-Pr-Box (L3) was applied, a 4:1 mixture of 2a and 3a was observed where 2a was formed in 57% ee (entry 3). Catalyst loading had a more significant impact on the ratio of products. Increased catalyst loading decreased the ratio of 2a:3a, tending to form more 3a as the loading increased (entries 6-8). At 60 mol % [Cu] loading, the ratio of products was close to 1:1. At 25 mol% [Cu] loading, using (S,S)-t-Bu-Box (L2), the ratio of 2a to 3a was 5:1; bis(ether) 3a was isolated and its enantiomeric excess was measured at 86% (entry 6). Changing the oxidant to Ag2CO3 did not strongly impact the reaction while addition of KMnO4 (10 mol%) increased the amount of bis(ether) 3a produced (entries 10 and 11). While the reaction worked in both toluene and DCE (1,2-dichloroethane, entries 12 and 13), neither reaction was superior to the reaction in α,α,α-trifluorotoluene. Cu(NTf2)2 was tried as an alternative source but was found to give lower 2a:3a selectivity for substrate 1a (entry 14). The ratio of 2a and 3a is kinetically controlled; both products were re-subjected to the reaction conditions and were not observed to interconvert.
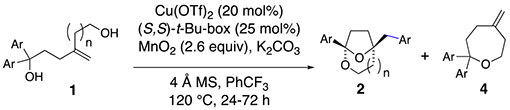
1,1-Diaryl-4-methylene-1,6-hexanediols bearing various aryl substituents were next examined in the enantioselective [3.2.1]-bridged bicyclic ketal synthesis (Table 2). While the parent 1,1-diphenyl substrate gave ketal 2a in 64% yield and 92% ee, 4- and 3-toluyl analogs gave ketals 2b and 2c in moderate yields (40-42%) but good enantioselectivities (87% and 92%, respectively, Table 2, entries 2 and 3). The remainder of the mass was attributed to oxepanes 4, formed via a competing SN1 process enabled by presumably more stabilized benzylic carbocations. Under the standard conditions, oxepane 4e was the predominant product (61% yield) with bis(4-methoxyphenyl) hexanediol 1e as substrate (Table 2, entry 6). By changing the catalyst to Cu(NTf)2, we were able to obtain ketal 2e in a modest 40% isolated yield and in 70% ee (Table 2, entry 7). Substrates with electron-withdrawing substituents fared better in the ketal synthesis reaction. 4-Trifluoromethylphenyl, 4-chlorophenyl, 3-chlorophenyl and 4-sulfonamidophenyl substituted hexane diols underwent the cyclization in 52-73% yield and with 88-97% ee (entries 4, 8, 9 and 10). Reaction of the 4-trifluoromethylphenyl substituted hexane diol was performed on 1 mmol scale (entry 5). Surprisingly, a 4-phenylbenzene substrate gave ketal 2i in 59% yield but with only 20% ee under standard conditions (entry 11). The enantioselectivity was improved to 56% ee by changing to the less sterically demanding (S,S)-i-Pr-Box ligand (L3) (entry 12). Ketal 2i was crystalline and its X-ray structure enabled its rigorous structural assignment (see Supporting Information for details). Reaction of 4-methylene-1,1-diphenylheptane-1,7-diol, whose more substituted alcohol chain is more conformationally pre-disposed toward cyclization, led to the [4.2.1]-bridged bicyclic ketal 2j, (entry 13).
Table 2.
Arene substituent scope and variable tether lengtha
 | |||||
|---|---|---|---|---|---|
| Entry | Ar | n | Product | yield (%)b | ee (%)c |
| 1 | Ph | 1 | 2a | 64 | 92 |
| 2d | 4-MeC6H4 | 1 | 2b | 40 | 87 |
| 3d | 3-MeC6H4 | 1 | 2c | 42 | 92 |
| 4e | 4-CF3C6H4 | 1 | 2d | 71 | >95 |
| 5f | 4-CF3C6H4 | 1 | 2d | 51 | >95 |
| 6 | 4-MeOC6H4 | 1 | 4e | 61 | -- |
| 7d,g | 4-MeOC6H4 | 1 | 2e | 40 | 70 |
| 8 | 4-ClC6H4 | 1 | 2f | 53 | 88 |
| 9g | 3-ClC6H4 | 1 | 2g | 58 | 94 |
| 10 | 4-TsMeNC6H4 | 1 | 2h | 73 | 90 |
| 11 | 4-PhC6H4 | 1 | 2i | 59 | 20 |
| 12h | 4-PhC6H4 | 1 | 2i | 64 | 56 |
| 13 | Ph | 2 | 2j | 68 | 93 |
Table 1, entry 1 conditions were applied with 0.1 mmol of substrate 1.
Isolated yield of major product following chromatography on silica gel.
Enantiomeric excess measured by chiral HPLC.
30-40% of an SN1 product 4 was also formed.
25 mol% Cu(OTf)2 and 31 mol% (S,S)-t-Bu-Box was used.
1 mmol of 1d was used. 34% of starting 1d was recovered.
Cu(NTf2)2 was used.
(S,S)-i-Pr-Box was used, reaction temperature was 105 °C (at 120 °C, 58% of 2i was obtained in 50% ee).
1,1-Diynyl-4-methylene-1,6-hexane diols readily underwent the cyclization and group transfer process to provide bridged bicyclic ketals 2k and 2l in moderate yield and very good enantioselectivity (Scheme 2). The absolute configuration of 2l was assigned by X-ray crystallography.
Scheme 2.
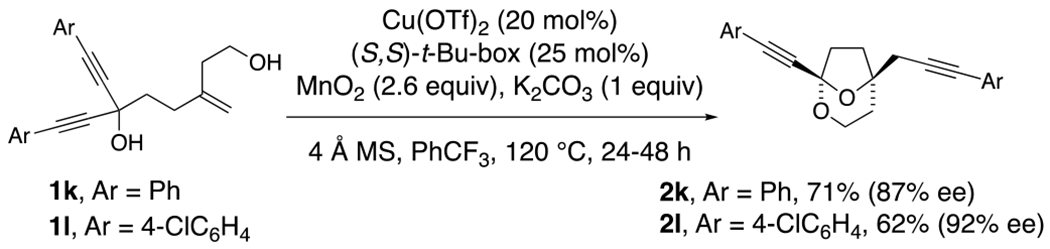
Alkyne-substituted examples
1,1-Diaryl-4-methylene-1,5-pentane diols 1m and 1n were investigated for their ability to form [2.2.1]-bridged bicyclic ketals (Scheme 3). Surprisingly, the 1,1-diphenyl substrate 1n exclusively formed spirooxetane 3m.
Scheme 3.

Reactions of allylic alcohols
Conversely, bis(biaryl) allylic alcohol 1n provided the [2.2.1]-bicyclic ketal exclusively. Ketal 2n was not stable under enantiomeric excess measurement conditions (chiral HPLC or GC) and oxetane 3m is achiral, so the reaction with the achiral bis(oxazoline) L1 is shown for these examples.
A mechanistic analysis is illustrated in Scheme 4. Complexation of the [Cu(II)] catalyst to the tertiary alcohol leads to intermediate I, which can enter the oxycupration TS-A, generating tetrahydrofuran II enantioselectively. In this transition state, the alkene terminal carbon approaches the copper anti to the t-Bu group on the nearest oxazoline ring.28 Organocopper intermediate II undergoes C-[Cu(II)] homolysis to give the carbon radical III. Carbon radical III can undergo ipso aryl addition, to give IV, which undergoes subsequent C-C bond cleavage/group transfer to give the alkoxy-stabilized carbon radical V. Oxidation of V then gives oxonium ion VI and subsequent ketalization provides bicyclic ketal 2 (Scheme 4A). A potential path for diminished enantioselectivity is ring opening of carbon radical III to give alkoxy radical VII, which could cyclize to give a racemic tetrahydrofuranyl carbon radical (±)-III (Scheme 4B).27 Alternatively, homolysis of the RO-[Cu(II)] bond of intermediate I could also give alkoxy radical VII. Substrates with resonance-donating aryl substituents (e.g. 1d, R = Ph and 1e, R = OMe, Scheme 4) give products 2d and 2e in lower enantioselectivity (Table 2) compared to substrates with neutral or electron-withdrawing substituents (e.g. 1a, R = H, 1d, R = CF3, Scheme 4 and Table 2). Electron-donating arene substituents could lower the oxidation potential of the nearby alcohol.34
A route to bis(ether) 3a involves carbon radical III combining with [Cu(II)] to give [Cu(III)] intermediate VIII. Coordination to the pendant alcohol then gives IX, and reductive elimination provides 3a.32–33 The rate of conversion of VIII to IX should increase with decreasing carbon tether length (forming 5- vs. 6-membered chelate) which could explain why allylic alcohol 1m forms the bis(ether) 3m (Scheme 3) while the homoallylic alcohol 1a forms ketal 2a (Tables 1 and 2).
That the bis(biaryl) allylic alcohol 1n forms ketal 2n, while the diphenyl allylic alcohol 1m forms oxetane 3m may be due to a faster rate of ipso addition to the phenyl-substituted arene, generating a more stabilized aryl radical intermediate.25
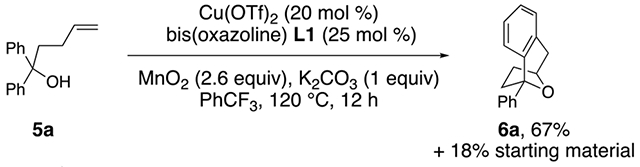
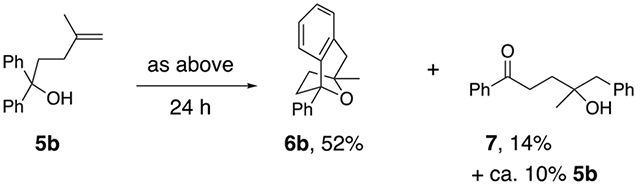
Substrates 5 that lack the second alcohol form bridged bicyclic [3.2.1] heterocycles 6. Terminal alkene 5a provides adduct 6a in 67% yield (Eq. 1).27 The 1,1-disubstituted alkene 5b, differing from 5a only in alkene substitution, provided adduct 6b and 14% of ketone 7 (Eq. 2). Ketone 7 is likely formed via group transfer and subsequent hydrolysis of the resulting intermediate. The higher substitution of 5b results in greater bond angle compression of its carbon radical intermediate, placing the radial closer to the arene ipso carbon, leading to 7.
In conclusion, a new route to enantioenriched bridged bicyclic ketals from achiral, acyclic alkenols has been developed. The polar / radical cross-over mechanistic abilities of copper (II) catalysis enable both polar enantioselective alkene addition and radical-based group transfer reactivity. Some reactivity trends associated with radical stability have been identified; these observations can be used to guide new reaction design.
 |
(1) |
 |
(2) |
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (GM078383) for support of this work and Prof. Jason Benedict for assisting E.D.S. with the X-ray structures of 2i and 2l.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/d0cc06404a
Conflicts of interest
There are no conflicts to declare.
Notes and references
Crystal structures of 2i and 2l have been deposited in the Cambridge Database, CCDC 2020673 and 1978089.
- 1.Bera S, Chatterjee B, Mondal D, Eur. J. Org. Chem 2018, 5337. [Google Scholar]
- 2.Bergstrom JD, Kurtz MM, Rew DJ, Amend AM, Karkas JD, Bosterdor RG, Bansal VS, Dufresne C, VanMiddlesworth FL, Hensens OD, Proc. Natl. Acad. Sci 1993, 90, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams PG, Asolkar RN, Kondratyuk T, Pezzuto JM, Jensen PR, Fenical W, J. Nat. Prod 2007, 70, 83. [DOI] [PubMed] [Google Scholar]
- 4.Mascitti V et al. J. Med. Chem 2011, 54, 2952. [DOI] [PubMed] [Google Scholar]
- 5.Milroy L, Zinzalla G, Loiseau F, Qian Z, Prencipe G, Pepper C, Fegan C, Ley SV, Chem Med Chem 2008, 3, 1922. [DOI] [PubMed] [Google Scholar]
- 6.Dawson MJ, Baxter A, Tait RM, Watson NS, Noble D, Shuttleworth A, Wildman HG, Hayes MV, WO9212156 A1, 1992.
- 7.Wilson MC, Gulder TAM, Mahmud T, Moore BS, J. Am. Chem. Soc 2010, 132, 12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson I, Razzak M, Anderson EA, Org. Lett 2008, 10, 3295. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, De Brabander JK, J. Am. Chem. Soc 2009, 131, 12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh CH, Yi HJ, Lee JH, New J Chem 2007, 31, 835. [Google Scholar]
- 11.Das S, Induvadana B, Ramana CV, Tetrahedron 2013, 53, 45. [Google Scholar]
- 12.Hirata Y, Nakamura S, Watanabe N, Kataoka O, Kurosaki T, Anada M, Kitagaki S, Shiro M, Hashimoto S, Chem. Eur. J 2006, 12, 8898. [DOI] [PubMed] [Google Scholar]
- 13.Cala L, Fananas FJ, Rodriguez F, Org. Biomol. Chem 2014, 12, 5324. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton JY, Rossler SL, Carreira EM, J. Am. Chem. Soc 2017, 139, 8082. [DOI] [PubMed] [Google Scholar]
- 15.Yoneda N, Fukata Y, Asano K, Matsubara S, Angew. Chem. Int. Ed 2015, 54, 15497. [DOI] [PubMed] [Google Scholar]
- 16.Midya A, Maity S, Ghorai P, Chem. Eur. J 2017, 23, 11216. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Chen F, Qu M, Li T, Liu Y, Shi M, Chem. Commun 2013, 49, 3360. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Lv X, Pei J, Tan R, Liu Y, Org. Chem. Front 2020, 7, 292. [Google Scholar]
- 19.Balha M, Pan SC, J. Org. Chem 2018, 83, 14703. [DOI] [PubMed] [Google Scholar]
- 20.Paz BM, Klier L, Naesborg L, Lauridsen VH, Jensen F, Jorgensen KA, Chem. Eur. J 2016, 22, 16810. [DOI] [PubMed] [Google Scholar]
- 21.Polat MF, Hettmanczyk L, Zhang W, Szabo Z, Franzen J, ChemCatChem 2013, 5, 1334. [Google Scholar]
- 22.Chan CK, Tsai YL, Chang MY, Org. Lett 2017, 19, 1870. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Gu Q, Wang N, Song P, Li Z, Li X, Wangand F, Liu X, Chem. Commun 2017, 53, 4038. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Xu W, Xie J, Yu S, Zhu C, Chem. Soc. Rev 2018, 47, 654. [DOI] [PubMed] [Google Scholar]
- 25.Fang J, Dong WL, Xu GQ, Xu PF, Org. Lett 2019, 21, 4480. [DOI] [PubMed] [Google Scholar]
- 26.Li L; Li ZL; Wang FL; Guo Z; Cheng YF; Wang N; Dong XW; Fang C; Liu J; Hou C; Tan B; Liu XY, Nat. Commun 2016, 7, 13852, DOI: 10.1038/ncomms13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller Y, Miao L, Hosseini AS, Chemler SR, J. Am. Chem. Soc 2012, 134, 12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bovino MT, Liwosz TW, Kendel NE, Miller Y, Tyminska N, Zurek E, Chemler SR, Angew. Chem. Int. Ed 2014, 53, 6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen D, Chemler SR, Org. Lett 2018, 20, 6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karyakarte SD, Um C, Berhane IA, Chemler SR, Angew. Chem. Int. Ed 2018, 57, 12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sequeira FC, Chemler SR, Org. Lett 2012, 14, 4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayoral JA, Rodriguez-Rodriguez S, Salvatella L, Chem. Eur. J 2008, 14, 9274. [DOI] [PubMed] [Google Scholar]
- 33.Huffman LM, Casitas A, Font M, Canta M, Costas M, Ribas X, Stahl SS, Chem. Eur. J 2011, 17, 10643. [DOI] [PubMed] [Google Scholar]
- 34.Yayla HG, Wang H, Tarantino KT, Orbe HS, Knowles RR, J. Am. Chem. Soc 2016, 138, 10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


