Abstract
Background
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-COV2) depends on RNA-dependent RNA polymerase (RdRp) enzyme complex for its genomic replications and thus can be inhibited by nucleoside analogues. An example is Remdesivir, which is a non-obligate chain terminator of RdRp. Therefore, we investigate the activities of Remdesivir against COVID-19.
Method
This is a systematic-review and meta-analysis of the literature on the effectiveness of Remdesivir in the management of COVID-19 through MEDLINE (from Jan 2019 to January 2021), EMBASE (from Jan 2019 to January 2021), Publics Ovidius Naso (Ovoid), Database of Abstracts of Reviews of Effects and the Cochrane Central Register of Controlled Trials in Issue 1 of 12, January 2021. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was applied and the questions generated in conformity with the participants, interventions, comparisons, outcomes, and study design (PICOS). Statistical analysis was performed in Stata v. 12.1 (StataCorp, Texas USA).
Results
The outcome of the reviewed relevant journals and the cross-references including clinical trials, systematic reviews and metanalysis were documented. Out of 569,000 articles, 11 roundly-suited the inclusion criteria. The comparative effects of Remdesivir on death (OR = 0.79; 95% CI = 0.57, 1.08) and recovery (OR = 2.22; p5% CI = 1.80, 2.73) were calculated.
Conclusion
Remdesivir is useful in the treatment of COVID-19 especially the severe disease. However, it should be used with caution since all the adverse effects are not known. We recommend Remdesivir as an alternative/third-force in the treatment of severe and critical COVID-19.
Keywords: COVID-19, Remdesivir, Third-force
1. Introduction
1.1. Structure of SARS-COV-2
The Corona virus disease 2019 (COVID-19) is caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV2), an envelope positive-sense-single stranded RNA virus belonging to the family of Coronaviridae, the order Nidovirales, and the genus Coronavirus (Fig. 1). These large group of viruses cause respiratory and gastrointestinal infections [1]. Coronaviruses are categorized into four important genera namely Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. A novel member of the human corona virus emerged in Wuhan, China, formally known as SARS-CoV-2 is responsible for the COVID-19 respiratory disease and capable of progressing to viral pneumonia and acute respiratory distress syndrome (ARDs).
Fig. 1.
Illustration of SARS-COV 2. This shows the different features of the virus including the spike, matrix, envelope and nucleocapsids proteins.
SARS-COV2 depends on RNA-dependent RNA polymerase (RdRp) enzyme complex for its genomic replications and thus can be inhibited by the class of drugs called nucleoside analogues. Remdesivir (GS-5734), is an example of this drug, it is an adenosine triphosphate described in 2016 as potential therapy for Ebola disease [2]. Remdesivir has demonstrated broad antiviral invitro activities against Coronaviridae family, Arenaviridae, Filoviridae, Flaviviridae, Paramyxoviridae etc [3].
Since 2017, Remdesivir was confirmed as a non-obligate chain terminator of RdRp against other related SARS-CoV and MERS-CoV, and has been investigated in multiple COVID-19 clinical trials [4]. Till date, it is the only antiviral agent that has been approved by FDA for the treatment of COVID-19 infection initially via emergency use authorization before a full approval was given on the 22nd October 2020 [5,6].
Remdesivir (Fig. 2) is indicated for patients diagnosed of COVID-19 infection from age 12 years and weighing 40 kg or more. It is known that Remdesivir carries risks for hypersensitivity reactions, including anaphylaxis and other infusion-related reactions, elevated transaminase levels, and has decreased efficacy when combined with hydroxychloroquine or chloroquine. Remdesivir is most indicated for moderate to severe and critical COVID-19 infection with SpO2 less than 94%. A loading intravenous dose of 200 mg must be given on the first day advisably in a hospital setting and under medical supervision to avert untoward events. Subsequently, intravenous dose of 100 mg should be given daily up to day 5 and maximum of day 10 depending on the severity of disease [7,8]. Remdesivir is a phosphonamidite metabolite of a 1′-cyano-substituted adenosine nucleotide analogue that competes with ATP for incorporation into the synthesized viral RNA by the corresponding RdRp complex [1]. It gains entry to the cells before being cleaved to its monophosphate form by carboxylesterase 1 or cathepsin A. It is phosphorylated by undescribed kinases to yield its active triphosphate form Remdesivir triphosphate (RDV-TP or GS-443902). Experiments suggest that at i + 4 (corresponding to the position for the incorporation of the fourth nucleotide following RDV-TP incorporation), the 1′-cyano group of Remdesivir sterically clashes with Ser-861 of the RdRp, preventing further enzyme translocation and terminating replication at position i + 3. This mechanism of action is essentially identical in SARS-CoV, SARS-CoV-2, and MERS-CoV [8].
Fig. 2.
The Molecular structure of Remdesivir. Illustrates the salient features of Remdesivir including the hydrocarbon arrangements that enhances its efficacy.
1.1.1. Review objectives-
Given the global urgency of the need for effective treatment for SARS-CoV-2 infection and the beneficial potential of Remdesivir, this systematic review and meta-analysis was performed to assess the risk of COVID-19 related deaths and adverse effects of the antiviral drug using accessible data from published studies. This review answers two questions of clinical interest in PICO (patient-intervention-comparison-outcome) format; will Remdesivir treatment minimise the risk of death among COVID-19 patients within 28 days of initiation of treatment compared to standard care? and Does the administration of Remdesivir give better recovery to COVID-19 patients within 28 days of initiation of treatment compared to standard treatment?
1.1.2. Protocol and search strategy-
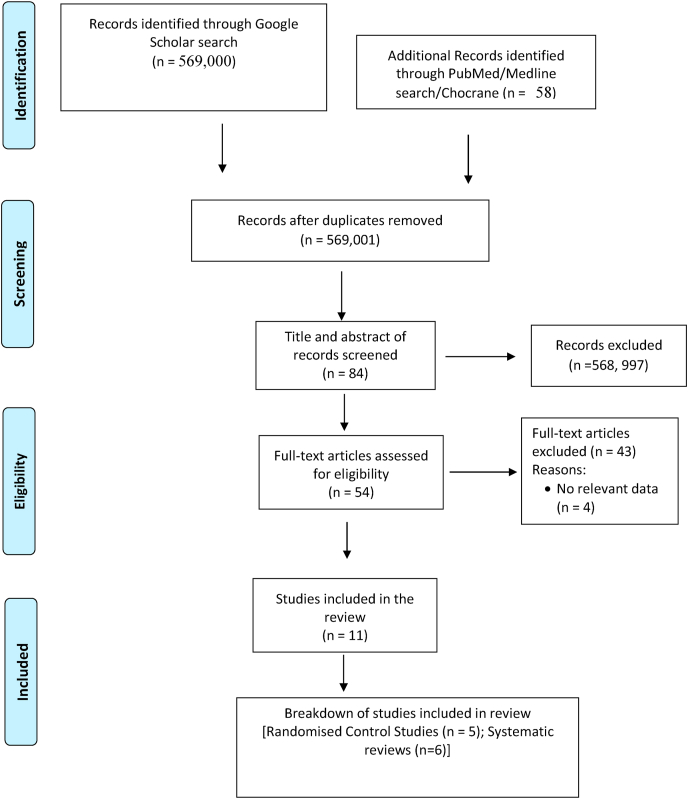
The protocol for this systematic review is being considered for approval and registration with PROSPERO (NIHR submission number: ID 240231). A comprehensive search of MEDLINE (from Jan 2019 to January 2021), EMBASE (from Jan 2019 to January 2021), Publics Ovidius Naso (Ovoid), Database of Abstracts of Reviews of Effects and the Cochrane Central Register of Controlled Trials in Issue 1 of 12, January 2021 of the Cochrane Library was performed. The search strategy used the exploded Medical Subject Headings term “Remdesivir” OR “Nucleoside analogue” OR “Anti-COVID-Antivirus drug” combined with the second set obtained using the exploded terms “treatment of COVID-19”. Reference lists and citation indexes of identified manuscripts were cross-referenced to identify further relevant literatures. Short-listing of titles and abstracts based on their relevance to the review and subsequent data extraction were undertaken independently by the two authors (TSI and PUI). The search range/review period was set to be from the declaration of COVID-19 as a pandemic by the World Health Organization (January 2020) to the time of the study (13th February 2021). No language restrictions were applied to ensure wide coverage in relevant publications. The steps taken in the selection of articles was illustrated in Fig. 3 algorithms.
Fig. 3.
Algorithms of articles selected for the study. The steps and processes for search, evaluation, filtration and final selections of articles used in the systematic review are shown.
1.1.3. Eligibility criteria and study selection
The studies found after the search were screened for eligibility based on types of studies, types of participants, types of interventions and outcomes. We selected and included studies that utilised parallel randomised controlled trials (RCTs) design regardless of language and status of publication, which stipulated that the length of Remdesivir administration was at least 5-days (Table 1). Two articles written in Spanish and German met the criteria for inclusion, and they were translated and included. Irrespective of the presence of comorbidity, we included RCTs involving adults with COVID-19. We found that intervention was particularly important for those individuals/groups with medical conditions or special care needs, so we incorporated the trials that involved those participants. The papers selected for inclusion in the analysis of Remdesivir as an interventional product included several studies that examined Remdesivir in relation to standard care. Only reports that recorded deaths and/or clinical rehabilitation that occurred on day-14 and day-28 after initiation of treatment were included in the meta-analysis.
Table 1.
Characteristics of selected articles for systematic review in “A third force in Management of COVID-19”.
| S/No | Categories | Authors | Purpose | Samples | Key findings | Level of Evidence |
|---|---|---|---|---|---|---|
| 1 | Randomised Clinical Trials (RCT) |
Spinner et al. (9) | To determine the efficacy of 5 or 10 days of Remdesivir treatment compared with standard care on clinical status on day 11 after initiation of treatment. | USA, Europe & Asia | N = 586/596 (numerator-completed; Denominator-Started) Remdesivir demonstrated strong clinical benefit in a placebo-controlled trial in patients with severe COVID-19 with efficacy best on days 5 and 10; but its effect on moderate disease is not known. Clinical status on day 11, was not significantly different from day 5 and 10 ((P = 0.18 by Wilcoxon rank sum test). |
2 |
| 2 | Randomised Control Trials |
Beigel et al. (12) | To assess the efficacy of Remdesivir on SARS-COV-2 infections. | USA, UK, Germany, Spain, Denmark, Japan, Korea, Mexico and Singapore | N = 1062 Those who received Remdesivir had a median recovery time of 10 days (95% confidence interval [CI], 9 to 11), as compared with 15 days (95% CI, 13 to 18) observed among those who received placebo (rate ratio for recovery, 1.29; 95% CI, 1.12 to 1.49; P < 0.001, by a log-rank test). Remdesivir was superior to placebo in shortening the time to recovery in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection. |
2 |
| 3 | Randomised Controlled Trials |
Wang et al. (13) | To analyse the efficacy of Remdesivir on COVID-19. | China | N = 237 RCT involving 237 patients (158 to Remdesivir and 79 to placebo) revealed no difference in time to clinical improvement (hazard ratio 1·23 [95% CI 0·87-1·75]). Although, not statistically significant, the patients that received Remdesivir had a numerically faster time to clinical improvement than those that received placebo among patients with symptom duration of 10 days or less (hazard ratio 1·52 [0·95-2·43]) |
2 |
| 4 | Randomised Control Trial | Goldman et al. (14) | To assess the efficacy of Remdesivir on COVID-19 | USA, UK, Germany, Spain, Denmark, Japan, Korea, Mexico and Singapore | N = 397 The patients underwent randomization and began treatment (200 patients for 5 days and 197 for 10 days). At baseline, patients randomly assigned to the 10-day group had significantly worse clinical status than those assigned to the 5-day group (P = 0.02). By day 14, a clinical improvement of 2 points or more on the ordinal scale occurred in 64% of patients in the 5-day group and in 54% in the 10-day group.In patients with severe Covid-19 not requiring mechanical ventilation, the trial did not show a significant difference between a 5-day course and a 10-day course of Remdesivir. |
|
| 5 | Randomised Control Trials |
Grein et al. (15) | To investigate efficacy of Remdesivir in compassionate use on patients | United States, China, France, Germany, Hong Kong, Italy, Japan, Korea, the Netherlands, Singapore, Spain, Sweden, Switzerland, Taiwan and the United Kingdom. | N = 53/61The patients whose data were analysed, 22 were in the United States, 22 in Europe or Canada, and 9 in Japan. At baseline, 30 patients (57%) were on mechanical ventilation and 4 (8%) were on extracorporeal membrane oxygenation.At median follow-up of 18 days, 36 (68%) improved on oxygen-support, including the extubated 17 of 30 patients (57%) on mechanical ventilator. Twenty-five patients (47%) got discharged, 7 (13%) died. Overall mortality was 18% (6 of 34) of those on active ventilation and 5% (1 of 19) of those not on ventilation. | 2 |
| 6 | Systematic Review |
Rochwerg et al. (16) | To provide clinical guide on management of Severe COVID-19 with Remdesivir. | Global | N = 13000 The study was based on the RCTT1 trial on Remdesivir. The guideline panel makes a weak recommendation for the use of Remdesivir in severe covid-19 while recommending continuation of active enrolment of patients into ongoing randomised controlled trials examining Remdesivir. |
1 |
| 7 | Systematic Review |
Piscoya et al. (17) | To investigate the efficacy and safety of Remdesivir for the treatment of COVID-19 | Global | N = 22960 Four randomized controlled trials (RCTs) (n = 2296) [two vs. placebo (n = 1299) and two comparing 5-day vs. 10-day regimens (n = 997)], and two case series (n = 88). Studies used intravenous Remdesivir 200 mg the first day and 100 mg for four or nine more days. One RCT (n = 236) was stopped early due to AEs (Adverse effects); the other three RCTs reported outcomes between 11 and 15 days. Time to recovery was decreased by 4 days with Remdesivir vs. placebo in one RCT (n = 1063), and by 0.8 days with 5-days vs. 10-days of therapy in another RCT (n = 397). Clinical improvement was better for 5-days regimen vs. standard of care in one RCT (n = 600). Remdesivir did not decrease all-cause mortality (RR 0.71, 95% CI 0.39 to 1.28, I2 = 43%) and need for invasive ventilation (RR 0.57, 95%CI 0.23 to 1.42, I2 = 60%) vs. placebo at 14 days but had fewer SAEs; 5-day decreased need for invasive ventilation and SAEs vs. 10-day in one RCT (n = 397). No differences in all-cause mortality or SAEs were seen among 5-day, 10-day and standard of care |
1 |
| 8 | Systematic Review | Yokoyama et al.(18) | To compare the rate of clinical improvement among patients with COVID-19 that received 5-day course of Remdesivir with 10-day course and standard care | Global | The meta-analysis of 4 RCTs showed a significant clinical improvement higher in the 5-day Remdesivir group and 10-day Remdesivir group compared to standard care group (OR [95% confidence interval [CI]] = 1.89 [1.40–2.56], P < 0.001, OR [95% CI] = 1.38 [1.15–1.66], P < 0.001, respectively). Clinical improvement was significantly higher in the 5-day Remdesivir group compared to the 10-day Remdesivir group (OR [95% confidence interval [CI]] = 1.37 [1.01–1.85], P = 0.041). Therefore, the use of Remdesivir for COVID-19 treatment was associated with the significantly higher clinical improvement rate compared with standard care alone. | 1 |
| 9 | Systematic review | Verdugo-Paiva et al.(19) | To assess the role of Remdesivir in the treatment of patients with COVID-19 | Israel, Iran, China, Japan, Korea, Australia, New-zealand, Brazil, Lebanon, Pan-Africa, Netgerlands, Srilanka, India, Germany | N=Not AvailableEffect of Remdesivir on mortality is uncertain (RR 0.7, 95% CI 0.46 to 1.05; very low certainty evidence) and relevance of invasive mechanical ventilation (RR 0.69, 95% CI 0.39 to 1.24; very low certainty evidence). Remdesivir appears associated with increase in adverse effects on COVID-19 patients (RR 1.29, 95% CI 0.58 to 2.84; moderate certainty evidence). | 1 |
| 10 | Systematic review | Nasir et al.(20) | To assess the evidence for efficacy and safety in the compassionate use of Remdesivir in severeCOVID-19 as re-purposeful use. | China, USA. UK and KSA | N = 523 Seven articles were reviewed in the current systematic review.The safety and efficacy of Remdesivir in COVID-19 cases requires high-quality evidence from well-designed and adequately-powered clinical trials with proper sample size for precise decision. |
1 |
| 11 | Systematic review | Alegre-Del et al.(21) | To analyse the reliability and clinical applicability of subgroup findings on the effect of Remdesivir on mortality in patients with COVID-19 | Global | N = 53/61 A validated tool was used to assess the findings of subgroup analyses in randomized clinical trials, including meta-analysis annexed to the SOLIDARITY study. This study suggests too much uncertainty in the hypothesis that Remdesivir could reduce mortality in patients with severe COVID-19 who require non-high flow oxygen. It elucidated that it might be a chance finding. More Randomised studies were recommended. |
1 |
We evaluated potentially eligible studies in line with the predefined selection criteria, and extracted data on study characteristics, methods, outcomes, and risk of bias, using a predesigned inclusion/exclusion criterion. The differences were resolved by mutual consensus. In addition, levels 1 and 2 evidenced publications (Systematic Reviews and Randomised Control Trials) on the efficacy and Adverse effects of Remdesivir in treating COVID-19 were included. All in-vitro, animal studies, review studies that failed to state the specific clinical outcomes and publications in newsletters, newspapers or under reviews were excluded. All relevant publications below level two scientific evidence were excluded to ensure quality and reliable outcome.
1.1.4. Data item and data collection process
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist was followed in conducting this review [9]. After searching and screening through all database records to locate relevant full-text articles, a data abstraction form was created. Two of the authors (TSI and PUI) performed data extraction, cross-checked the extracted data, and compiled the information using Microsoft Excel 2010. To make sure the extracted data is correct, the third author (AEO) used original data published in the selected complete texts to verify the data. Any errors were detected and fixed.
1.1.5. Assessment of quality of studies
We assessed the quality of selected studies and potential risk of bias using the “JBI Critical Appraisal Checklist for Randomized Controlled Trials” [10]. This process comprises 13 items that assess bias measurement, bias collection, bias analysis, and overall bias risk assessment. The decision to include, exclude or seek more details was based on the reviewer's assessment as either low, moderate, or high with reference to AMSTAR check-list 2 [11]. If there is not enough information to make a fair overall assessment, “no information” was allocated to the analysis and not used for data synthesis. Only studies with low to moderate overall bias risks were used for data synthesis.
1.1.6. Data synthesis and analysis
Two study authors separately and in duplicate collected data from the included studies using a personalised Excel spreadsheet that was checked on a limited sample of studies. We contacted the authors of the study for clarification or missing details where appropriate and feasible. We settled any disputes by negotiation and, where consensus could not be achieved, the third author agreed on the matter of concern. The data collected and reported included: study design, location/setting, number of centres, length of study, descriptions of participants including demographic characteristics, inclusion and exclusion criteria, and relevant information on baseline levels of Remdesivir, numbers randomised to each study arm, and numbers examined in each arm. Other key data collected are the number of participants, the form of experimental/comparator and the specifics of the findings recorded, including the process and timing of the evaluation. Since we anticipated significant clinical and methodological heterogeneity, we narratively summarized the potential effect of Remdesivir on death and recovery by days 14–28.
We performed a meta-analysis to pool data on death and recovery by days 14–28 as shown in Table 2, Table 3 and the forest plots (Fig. 4, Fig. 5). This is the only outcome with relatively well-recorded data from qualifying studies with a low or moderate risk of bias. Heterogeneity was tested using the Chi square-based Q statistic (significant for P < 0.1). The funnel plot and the Egger test were used to screen for small-study effects, a possible source of publication bias (Figures S1, S1a and S1b). Effect size results were based on an unadjusted odds ratio with a 95% confidence interval (CI). Statistical analysis was performed in Stata v. 12.1 (StataCorp, Texas USA) and used metan commands to generate summary estimates, forest and funnel plots (Figs. 4,5, S3).
Table 2.
Summary statistics of three studies included in the meta-analysis for the effect Remdesivir on death among COVID-19 patients.
| Study author | Remdesivir group |
Controls |
OR | 95% CI | ||
|---|---|---|---|---|---|---|
| Deaths | Survivors | Deaths | Survivors | |||
| Spinner et al., | 5 | 188 | 4 | 196 | 1.30 | 0.34, 4.93 |
| Beigel et al., | 59 | 482 | 77 | 444 | 0.71 | 0.49, 1.01 |
| Wang et al., | 22 | 136 | 10 | 68 | 1.10 | 0.49, 2.46 |
| M − H Pooled estimates | – | – | – | – | 0.79 | 0.57, 1.08 |
Test of pooled OR = 1; Z = −1.482; P = 0.138.
Table 3.
Summary statistics of three studies included in the meta-analysis for the effect Remdesivir on death among COVID-19 patients.
| Study author | Remdesivir group |
Controls |
OR | 95% CI | ||
|---|---|---|---|---|---|---|
| Better | Not better | Better | Not better | |||
| Spinner et al., | 185 | 8 | 86 | 114 | 30.65 | 14.32, 65.62 |
| Beigel et al., | 399 | 142 | 352 | 169 | 1.35 | 1.03, 1.76 |
| Wang et al., | 103 | 55 | 45 | 33 | 1.37 | 0.79, 2.39 |
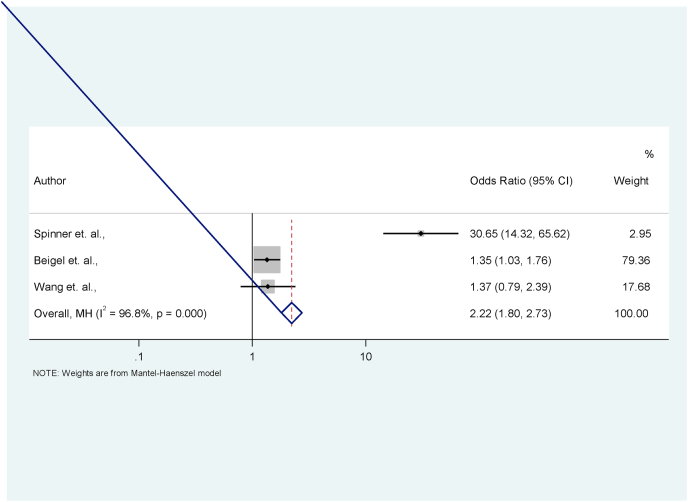
| M − H Pooled estimates | – | – | – | – | 2.22 | 1.80, 2.73 |
Test of pooled OR = 1; Z = 7.464; P < 0.001.
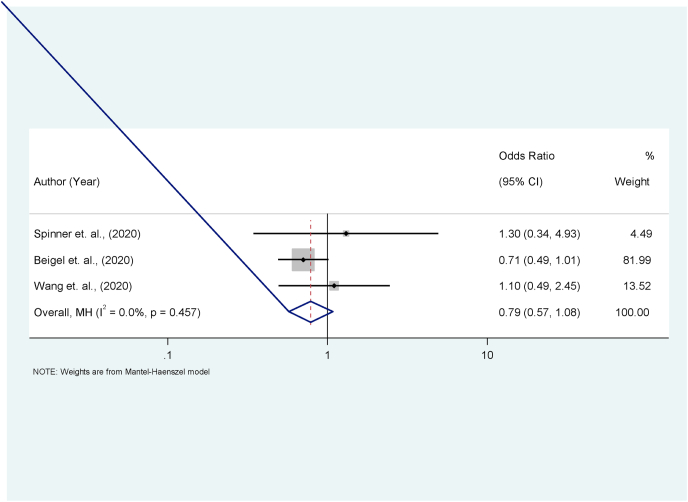
Fig. 4.
Forest plot for effect of Remdesivir on COVID-19 death. The actual effect and influence on the death rate of those with severe COVID-19 disease treated with Remdesivir was tested. There is a marginal advantage over death rate for the use of Remdesivir compared to standard method though not statistically significant.
Fig. 5.
Forest plot for the effect of Remdesivir on day 14–28. No significant advantage was recorded though numerical data suggests an advantage over 5-day therapy. More data is needed for the validation of this.
2. Results
2.1. Characteristics and summary of individual study findings
A total of 569,000 articles were returned following a wide search through the relevant search engines. They were eventually filtered down to 56 articles. Critical assessments unveiled eleven (11) studies that roundly met the inclusion criteria (5-Randomised Control trials and 6-Systematic reviews). The algorithm (Fig. 2) illustrates the steps in the screening of the articles.
A recent randomised clinical trial (NCT04292730) on Remdesivir demonstrated strong clinical benefit in a placebo-controlled trial in patients with severe Coronavirus disease but its effect on moderate disease is not known. Efficacy was well established in the 5th day and 10th day treatment group compared with the Standard treatment group across 105 hospitals in USA, Europe and Asia in a trial concluded in May 20, 2020 [12]. Out of 596 patients 584 enrolled in the study some with co-morbidities such as cardiovascular diseases and diabetes mellitus, statistically significant odds were recorded among the 5-day trial Remdesivir group over the Standard treatment group (OR 1.65, 95%% CI, 1,09-2,48; P = 0.02). The clinical status distribution on eleventh day for the 10day Remdesivir trial versus Standard group was not statistically significant (P = 0.18 Wilcox Rank sum test) [12].
In another publication, Remdesivir was given to patients with severe COVID-19 by Augustin et al. [13], it was noted that Remdesivir therapy demonstrated a significant clinical improvement in 68% of the patients with severe COVID-19 infection. At the same time, however, 23% of patients developed serious side effects (including multiple organ failure, septic shock, acute kidney injury, and hypotension). In the absence of a control group, it remains unclear whether Remdesivir was responsible for the clinical improvement or the side effects [13].
A clinical trial (NCT04280705) led by Beigel et al. [14] published in the New England Journal, showed the result of 1062 patients who underwent randomization with 541 assigned to Remdesivir and 521 to placebo. Those who received Remdesivir had a median recovery time of 10 days (95% confidence interval [CI], 9 to 11), as compared with 15 days (95% CI, 13 to 18) observed among those who received placebo (rate ratio for recovery, 1.29; 95% CI, 1.12 to 1.49; P < 0.001, by a log-rank test). In an analysis that used a proportional-odds model with an eight-category ordinal scale, the patients who received Remdesivir were found to be more likely than those who received placebo to have clinical improvement at day 15 (odds ratio, 1.5; 95% CI, 1.2 to 1.9, after adjustment for actual disease severity). The Kaplan-Meier estimates of mortality were 6.7% for Remdesivir and 11.9% for placebo group by day 15 and 11.4% for Remdesivir and 15.2% for placebo group by day 29 (hazard ratio, 0.73; 95% CI, 0.52 to 1.03). Serious adverse events were reported in 131 of the 532 patients who received Remdesivir (24.6%) and in 163 of the 516 patients who received placebo (31.6%) [13].
Furthermore, a rapid review of the anti-coronavirus-19 effect of Remdesivir by Li et al. [8] showed positive results in both laboratory experiments and reports from compassionate use, however its safety and effect in humans requires high-quality evidence from well-designed and adequately powered clinical trials for further clarification.
A randomised double-blind, placebo controlled multicentre trial (NCT04257656) on Remdesivir in adults with severe COVID-19, by Wang et al. [15] published in Lancet involving 237 patients (158 to Remdesivir and 79 to placebo) revealed no difference in time to clinical improvement (hazard ratio 1·23 [95% CI 0·87-1·75]). Although not statistically significant, patients receiving Remdesivir had a numerically faster time to clinical improvement than those receiving placebo among patients with symptom duration of 10 days or less (hazard ratio 1·52 [0·95-2·43]). Adverse events were reported in 102 (66%) of 155 Remdesivir recipients versus 50 (64%) of 78 placebo recipients. Remdesivir was stopped early because of adverse events in 18 (12%) patients versus four (5%) patients who stopped placebo early [15].
Another randomised open label phase-3 trial of Remdesivir in patients with severe COVID-19, including radiological evidence of pneumonia and oxygen saturation of 94% or less was conducted by Goldman et al. [16] and published in the New England Journal of Medicine. The result showed that 397 patients underwent randomization and began treatment (200 patients for 5 days and 197 for 10 days). At baseline, patients randomly assigned to the 10-day group had significantly worse clinical status than those assigned to the 5-day group (P = 0.02). By day 14, a clinical improvement of 2 points or more on the ordinal scale occurred in 64% of patients in the 5-day group and in 54% in the 10-day group. After adjustment for baseline clinical status, patients in the 10-day group had a distribution in clinical status at day 14 that was like that among patients in the 5-day group (P = 0.14) [16].
Grein et al. [17], conducted a randomised control study across United States, China, France, Germany, Hong Kong, Italy, Japan, Korea, the Netherlands, Singapore, Spain, Sweden, Switzerland, Taiwan and the United Kingdom. It was conducted among compassionate use patients (first set of volunteers for repurposing Remdesivir against COVID-19). Out of the 61 enrolees, 53 completed the study. At the median follow-up of 18 days, 36 (68%) improved on oxygen-support, including the extubated 17 of 30 patients (57%) on mechanical ventilator. Twenty-five patients (47%) got discharged, 7 (13%) died. Overall mortality was 18% (6 of 34) of those on active ventilation and 5% (1 of 19) of those not on ventilation [17].
A clinic practice guideline was developed on severe COVID-19 management with Remdesivir by Rochwerg et al. [18] in 2020. It was hinged on the ACTT-1 trial earlier published in the New England Journal of Medicine on 22 May 2020. The guideline panel made a weak recommendation for the use of Remdesivir in severe COVID-19 infection while recommending continuation of active enrolment of patients into ongoing randomised controlled trials examining Remdesivir. This body of evidence emerged through systematic reviews and metanalysis networks including two randomised trials in 1300 patients [18].
Beyond the Rochwerg et al. [18] study, five other systematic reviews on the efficacy of Remdesivir were retrieved from the literature search and analysed in detail (Table 1). These include the Piscoya et al. [19], Yokoyama et al. [20], Verdugo-Paiva et al. [21], Nasir et al. [22], and Alegre Delet al [23], respectively. The essence of their findings was tabulated in Table 1 (Nos 6–11).
2.2. Effect of Remdesivir on death among COVID-19 patients
The review of the three studies eligible for meta-analysis revealed that there were a total of 86 and 91 deaths recorded among the Remdesivir and control arms, respectively (Table 2). There was no significant difference in the odds of deaths among Remdesivir arm compared with control arm in each of these studies (Table 3). Also, the pooled estimation of the ratio of the size of the effect of Remdesivir compared with control (OR = 0.79; 95% CI = 0.57, 1.08) was not statistically significant (p = 0.128). In the same manner, the estimation of the size effect of Remdesivir compared with control for each study as well as the weight of the contributions of each study to the pooled estimate of the effect size are as shown in the forest plot (Fig. 4). Notably, the study by Beigel et al. [14] contributed the most (84.8%) to the observed effect. The proportion of total variance in effect estimate due to between-study heterogeneity (based on Q) was 0.0% and this was not statistically significant (p = 0.441).
2.3. Effect Remdesivir on recovery at day 14–28 among COVID-19 patients
Out of the three studies included in the meta-analysis, 687 out of 892 randomised in the Remdesivir arm compared with 483 out of 799 in the control arm had improved clinical status by day 14–28 after initiation of treatment (Table 3, Fig. S2). The pooled estimates of the effect size showed that patients randomised to the Remdesivir arm had 2.22 times higher odds of experiencing improved clinical status by day 14–28 than those in the control arm (OR = 2.22; 95% CI = 1.80 to 2.73). Also, the forest plot (Fig. 5) display the effect sizes of each of the three studies and their contributions to the pooled estimate of the effect size. The study by Beigel et al. [14] contributed an estimated 79.38% to the observed effect of Remdesivir. The proportion (96.8%) of total variance in estimated effect due to between-study heterogeneity (based on Q) was statistically significant (p < 0.001). No significant serious-adverse-events were recorded when compared with the standard treatment (Fig. S3).
2.4. Quality of included studies and publication bias
All the studies included in this review have low risk. The assessment for publication bias revealed no demonstrable bias in the distribution of the publications included in the meta-analysis on the effect size for mortality as shown in the Funnel plot (Fig. S1). Also, the Egger test demonstrated no small-study effect (Fig. S1a). However, the assessment of studies included in the meta-analysis for the evaluation of the effect on recovery showed significant bias as the study by Spinner et al. [12] falls considerably out the symmetry of the funnel plot. Nonetheless, the Egger test showed no significant small-study effect (Fig. S1b).
3. Discussion
Biochemical evidence has shown that SARS-COV2 (Fig. 1), depends on RNA-dependent RNA polymerase (RdRp) enzyme complex for its genomic replications and thus can be inhibited by the class of drugs called nucleoside analogues (Fig. 2) [1]. The most prominent and most widely studied nucleoside analogue remains Remdesivir. Its broad-spectrum inhibitory actions on coronaviruses have been demonstrated in vivo and in vitro with some success. Till date, it remains the only antiviral drug against COVID-19 that has received full approval for emergency use by FDA [5]. The pharmacodynamic and kinetics of Remdesivir have been well established. The dose is 200 mg stat and 100 mg daily given intravenously for 5–10days. Again, the desired weight of the patient is 40 mg and above. The availability of the drugs is best when given parenterally because of the poor gastrointestinal absorption [16].
The efficacy has been well demonstrated in severe and to some extent moderate diseases; which is where the benefit is usually eminent. Furthermore, there has been no obvious advantage of the extended 10-day therapy over the 5-day therapy regarding response to diseases within the context of time to discharge, hospital stay and achievement of asymptomatic state [12,14,16,19,20]. Compared to the standard therapy for COVID-19 which excludes Remdesivir; there is an obvious advantage in the use of the Remdesivir combination therapy. This is supported by some randomised, blind placebo trials on Remdesivir [12,[14], [15], [16], [17], [18]].
Even though the advantage of Remdesivir in severe and moderate diseases is established, the level of evidence in this prospect is not yet clear as demonstrated in the 5-out of the 6-sytematic reviews analysed (Table 1) in this study [18,19,[21], [22], [23]]. The voracity of complications associated with Remdesivir including the potential adverse effects/events are not fully defined [19,21,22]. These suggest that Remdesivir should be used with caution and only when strictly indicated as in severe COVID-19 infections. Furthermore, each patient under this therapy should be closely monitored for adverse events and idiosyncrasies [18,20].
The effect of Remdesivir on the reduction of deaths in the patients treated for COVId-19 showed no significant advantage over the conventional (non-Remdesivir based regime). Although, numerically there were less deaths recorded among the Remdesivir treated group compared to the patients treated with standard regime (86 vs 93), the Odd ratio and p-value did not show significant advantage in the reduction of death rate in severe/critical cases of COVID -19 infection within the first 28 days (Table 2 and Fig. 4). The reason for this apparent numerical reduction in death among Remdesivir treated patients may be related with the earlier documented clinical responses and advantages of Remdesivir in the 5th day of therapy compared to the standard treatment [9,[12], [13], [14]]. Shorter hospital stay has also been established by most RCTs in the Remdesivir group [[17], [18], [19]]. These two factors could have bestowed some advantages in the Remdesivir managed group, though not significant statistically but worthy of further investigations through well designed RCTs assessing the death rate in severe COVID -19 cases within the first 5 to 10-days.
The number of those who attained clinical recovery in 14- and 28-days post therapy in the Remdesivir group compared with the control group had both significant Odd ratio and p-values. This clinical recovery advantage among the Remdesivir group could be contributory in the overall outcome for survivors and mortalities which also strengthens the numerical non-statistical advantage in reduction in death from both severe and critical cases as shown earlier. All the RCTs [12,14,15] analysed in this study were in agreement with this finding.
Finally, we conclude that Remdesivir is useful in the treatment of COVID-19 especially the severe disease. However, it should be used with caution since all the adverse and untoward effects are not fully known. We recommend that Remdesivir be part of the regime for the management of COVID-19 in treatment centres to serve as an alternative/third force for the treatment of severe and complicated COVID-19.
3.1. Limitations
Given that COVID-19 is a trending topic, over 500,000 articles popped up during the search, thereby making assessment cumbersome. However, the assessors shared the articles for a painstaking assessment that finally yielded the ones used for the study. Very few RCTs of Remdesivir were available in the literature and thus used for analysis.
Ethical approval
Not Applicable.
Sources of funding
None.
Author contribution
1. Prof. Titus S Ibekwe(TSI)- Conceived the Research, participated in design, data collection, writing the paper, review and approval of Manuscript.
2. Dr. Perpetua U Ibekwe(PUI)- Participated in design, data collection, writing the paper, review and approval of Manuscript.
3. Prof. Emmanuel A Orimadegun(EAO)-Participated in the revision of the article, contributed in the systematic review and the statistical analysis.
Registration of research studies
-
1.
Name of the registry: PROSPERO
-
2.
Unique Identifying number or registration ID: NIHR: ID 240231
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): PROSPERO (york.ac.uk)
Guarantor
Prof. Titus S Ibekwe, Department of Otorhinolaryngology, University of Abuja Nigeria. PMB 117, Abuja Nigeria.
Provenance and peer review
Not commissioned, externally peer reviewed.
Declaration of competing interest
None.
Acknowledgement
Departments of Otorhinolaryngology and Dermatology unit UATH Abuja, for technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102218.
Contributor Information
Titus Ibekwe, Email: titus.ibekwe@uniabuja.edu.ng.
Perpetua Ibekwe, Email: perpetua.ibekwe@uniabuja.edu.ng.
Emmanuel Adebola Orimadegun, Email: bolaorimadegun@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gordon C.J., Tchesnokov E.P., Woolner E. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;15(20):6785–6797. doi: 10.1074/jbc.RA120.013679. 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malin J.J., Suarez I., Priesner V., Fatkenheuer G., Rybniker J. Remdesivir against COVID-19 and other viral diseases. Clin. Microbiol. Rev. 2020 Oct 14;(1):34. doi: 10.1128/CMR.00162-20. pii: 34/1/e00162-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aleem A., Kothadia J.P. StatPearls Publishing; 2020. Remdesivir. 2020 Oct 26. in: StatPearls [Internet]. Treasure Island (FL) Jan–. PMID: 33085408. Accessed. [Google Scholar]
- 4.FDA: Emergency Use Authorization for Baricitinib in Combination with Remdesivir for COVID-19 ([Link]).
- 5.Lamb Y.N. Remdesivir: first approval. Drugs. 2020 Sep;80(13):1355–1363. doi: 10.1007/s40265-020-01378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA approved drug products: VEKLURY (Remdesivir) injection.
- 7.Gordon C.J., Tchesnokov E.P., Woolner E. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;15(20):6785–6797. doi: 10.1074/jbc.RA120.013679. 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z., Wang X., Cao D., Sun R., Li C., Li G. Rapid review for the anti-coronavirus effect of Remdesivir. Drug Discov Ther. 2020;14(2):73–76. doi: 10.5582/ddt.2020.01015. [DOI] [PubMed] [Google Scholar]
- 9.Moher D., Shamseer L., Clarke M. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015 Jan 1;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tufanaru C., Munn Z., Aromataris E., Campbell J., Hopp L. Chapter 3: systematic reviews of effectiveness. In: Aromataris E., Munn Z., editors. Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute; 2017. https://reviewersmanual.joannabriggs.org/ Available from: [Google Scholar]
- 11.Shea B.J., Reeves B.C., Wells G. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017 Sep 21;358:j4008. doi: 10.1136/bmj.j4008. PMID: 28935701; PMCID: PMC5833365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spinner C.D., Gottlieb R.L., Criner G.J. GS-US-540-5774 investigators. Effect of Remdesivir vs standard care on clinical status at 11 Days in patients with moderate COVID-19: a randomized clinical trial. J. Am. Med. Assoc. 2020 Sep 15;324(11):1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augustin M., Hallek M., Nitschmann S. Remdesivir beiPatientenmitschwerer COVID-19 [Remdesivir for patients with severe COVID-19] Internist. 2020;61(6):644–645. doi: 10.1007/s00108-020-00800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beigel J.H., Tomashek K.M., Dodd L.E. ACTT-1 study group members. Remdesivir for the treatment of covid-19 - final report. N. Engl. J. Med. 2020;5(19):1813–1826. doi: 10.1056/NEJMoa2007764. 383, Epub 2020 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. Erratum in: Lancet. 2020. 30;395(10238):1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman J.D., Lye D.C.B., Hui D.S. GS-US-540-5773 investigators. Remdesivir for 5 or 10 Days in patients with severe covid-19. N. Engl. J. Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grein J., Ohmagari N., Shin D. Compassionate use of Remdesivir for patients with severe covid-19. N. Engl. J. Med. 2020 Jun 11;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rochwerg B., Agarwal A., Zeng L. Remdesivir for severe covid-19: a clinical practice guideline. BMJ. 2020 Jul 30;370:m2924. doi: 10.1136/bmj.m2924. [DOI] [PubMed] [Google Scholar]
- 19.Piscoya A., Ng-Sueng L.F., Parra Del Riego A. Efficacy and harms of Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. PloS One. 2020;15(12) doi: 10.1371/journal.pone.0243705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoyama Y., Briasoulis A., Takagi H., Kuno T. Effect of Remdesivir on patients with COVID-19: a network meta-analysis of randomized control trials. Virus Res. 2020;15:288. doi: 10.1016/j.virusres.2020.198137. 198137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verdugo-Paiva F., Acuña M.P., Solá I., Rada G. COVID-19 L·OVE Working Group. Remdesivir for the treatment of COVID-19: a living systematic review. Medwave. 2020 Dec 9;20(11) doi: 10.5867/medwave.2020.11.8080. English. [DOI] [PubMed] [Google Scholar]
- 22.Nasir M., Talha K.A., Islam T., Saha S.K., Selina F., Parveen R.A. Use of Remdesivir in the management of COVID-19: a systematic review on current evidences. Mymensingh Med. J. 2020 Apr;29(2):481–487. PMID: 32506110. [PubMed] [Google Scholar]
- 23.Alegre-Del Rey E.J., Gil-Sierra M.D., Alarcón de la Lastra-Romero C., Sánchez-Hidalgo M. Remdesivir and mortality reduction in COVID-19 patients: a systematized subgroup analysis of clinical trials. Farm. Hosp. 2021 13;45(1):28–31. doi: 10.7399/fh.11591. English. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.