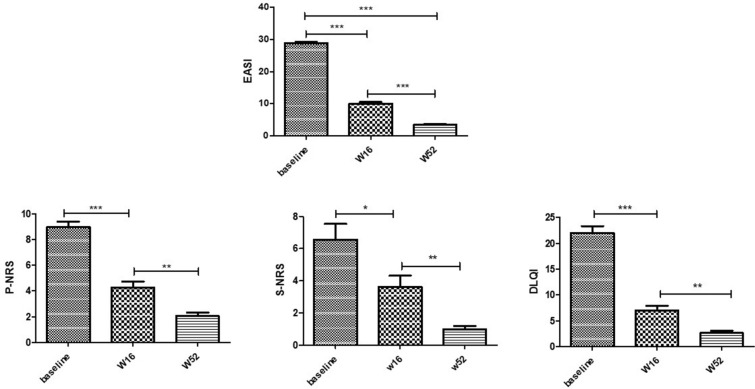
Fig. 1.
Improvement in terms of mean variation of EASI, P-NRS, S-NRS and DLQI from baseline to weeks 16 and 52. Mean values of EASI (Eczema Area and Severity Index), S-NRS and P-NRS (Sleep and Pruritus Numerical Rating Score) and DLQI (Dermatology Life quality Index) of study population before (week 0, W0) and after 16 weeks (W16) and W52 of dupilumab treatment. Statistical significance was assessed by the Mann–Whitney test and Fisher test: ***p < 0.0001, **p < 0.001, *p < 0.01