Abstract
Red dot basal cell carcinoma is a distinctive clinical subtype of basal cell carcinoma. It has been reported in eight individuals with a male to female ratio of 1:1; and the patients’ ages ranged from 50 to 74 years. All patients had prior history of actinic keratoses and basal cell carcinoma. In addition, some patients also had prior squamous cell carcinoma, malignant melanoma, and/or dysplastic nevus. The tumor was usually of recent onset, asymptomatic, and on sun-exposed skin. It was most commonly located on the nose (five patients); other sites were the upper lip, the mid back, or thigh—each in one patient. The red dot basal cell carcinoma was solitary and small—usually 4 mm or less in diameter. It typically presented as a red macule or papule; however, it sometimes appeared as a flesh-colored or pink to light-red papule with a bright-red central area. Microscopic features showed basaloid tumor cells (arranged as either nodular aggregates or superficial buds or both). In the central portion of the lesion, there was a proliferation of erythrocyte-containing vascular spaces between the epidermis and the neoplasm. The basal cell carcinoma pathology subtype was either nodular and superficial (three patients), nodular (two patients), or superficial (one patient). The clinical differential diagnosis of red dot basal cell carcinoma included not only benign vascular lesions (such as hemangioma and telangiectasia) but also inflammatory conditions and adnexal tumors. Other basaloid cell neoplasms were in the pathologic differential diagnosis. The pathogenesis of red dot basal cell carcinoma is similar to that of other basal cell carcinoma clinical subtypes. Mohs surgery is the treatment of choice for red dot basal cell carcinomas. Red dot basal cell carcinoma has two categories of biologic behavior based on the ratio of the postoperative wound size as compared with the size of the preoperative tumor: nonaggressive (for which the ratio was 5:1 or less for three patients) and aggressive (for which the ratio was greater than 12:1 for three patients). There was no recurrence of the red dot basal cell carcinoma after treatment. In conclusion, the incidence of red dot basal cell carcinoma—a unique morphologic variant of basal cell carcinoma—may be higher than suggested by the number of reported patients with this basal cell carcinoma subtype.
Keywords: Basal, Carcinoma, Cell, Dot, Hemangioma, Subtype, Telangiectasia, Red, Variant, Vascular
Key Summary Points
| Red dot basal cell carcinoma is a unique morphologic variant of basal cell carcinoma—usually of recent onset, asymptomatic, and on sun-exposed skin—that has been described in eight individuals who ranged in age from 50 to 74 years. |
| Red dot basal cell carcinoma is a small (less than 4 mm), solitary, red macule or papule that was located on the face (nose, five patients or upper lip, one patient), the mid back (one patient) or thigh (one patient). |
| Red dot basal cell carcinoma microscopically showed erythrocyte-containing vascular spaces in the center of the lesion between the epidermis and the dermal basaloid tumor cells. |
| Mohs surgery is the treatment of choice for red dot basal cell carcinoma. |
| The incidence of red dot basal cell carcinoma may be higher than suggested by the number of reported patients with this basal cell carcinoma subtype. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13625621.
Introduction
Red dot basal cell carcinoma is a unique morphologic variant of basal cell carcinoma. It has a distinctive clinical presentation. Red dot basal cell carcinoma has been described in eight individuals [1–4].
The purpose of this paper is to summarize the characteristics of patients with red dot basal cell carcinoma and the features of their tumor. These include the history, epidemiology, associated skin tumors, past medical history, duration, symptoms, location, clinical presentation, diagnosis, pathology, differential diagnosis, pathogenesis, and prognosis. Indeed, the prevalence of red dot basal cell carcinoma may be greater than that suggested by the number of published reports of this subtype of basal cell carcinoma.
This article is based on previously conducted studies and does not contain studies with human participants or animals performed by any of the authors. However, the patient signed a consent providing permission for including the clinical photographs in this article.
Discussion
History
The salient features of red dot basal cell carcinomas are summarized in publications listed in Table 1 [1–7]. To the best of the authors’ knowledge, the first description of red dot basal cell carcinoma was in a review article on unusual basal cell carcinomas published in 1994 [5]. The authors described the clinical presentation of the tumor and referred to the cancer as either a red dot basal cell carcinoma or a halo basal cell carcinoma [5].
Table 1.
Red dot basal cell carcinoma publications
| Authors Month, yeara |
Comment | References |
|---|---|---|
|
Johnson et al. August 1994 |
In the section on morphologic characteristics of unusual BCCs, red dot or halo BCC was mentioned. The authors commented that the tumor usually occurred on sun-exposed areas; its clinical appearance was a small (1–2 mm) red papule, sometimes with a halo. Early diagnosis required a high index of suspicion | [5] |
|
Tromberg et al. October 2012 |
The red dot BCC of two patients were described, and the features of this tumor were shared. The authors commented that red dot BCC is a distinct and early clinical presentation of BCC that they regularly encountered in their practice | [4] |
|
Loh and Cohen May 2016 |
A female patient with a red dot BCC on her distal left nasal bridge is described. The authors compare and discuss the clinical, diascopy, biopsy, and treatment features of red dot BCC and telangiectasia | [3] |
|
Cohen March 2017 |
Three patients with red dot BCC were described. The BCC was located on either the mid back (one female), the nasal tip (one male), or the nostril (one male). The characteristics of these patients and those of the previously reported four individuals with this clinical variant of BCC were reviewed | [2] |
|
Cohen May 2017 |
The paper, accepted for publication in December 2014, includes the description of a female with a red dot BCC on the distal lateral left thigh proximal to her knee. The author commented that it may be challenging to clinically differentiate red dot BCC from a similar appearing vascular lesion such as an angioma or a telangiectasia | [1] |
|
Cohen et al. September 2018 |
The paper is a patient and physician’s experience regarding BCC. In the physician’s perspective, red dot BCC is included the discussion and table of clinical types of BCC | [6] |
|
Cohen July 2019 |
A letter to the editor—in reply to a review article on BCC that had not mentioned red dot BCC—that emphasized the inclusion of red dot BCC as a unique clinical variant of BCC | [7] |
|
Cohen et al. 2021 |
A comprehensive literature review of red dot BCC; a new description of a red dot BCC on the right side of the patient’s upper lip is included in the figure legends | CR |
BCC basal cell carcinoma, CR current report
aMonth and year of publication
In October 2012, 18 years later, a description of two patients with red dot basal cell carcinoma was reported. However, the investigators were not aware of the previously published paper on this neoplasm. They commented that, in their practice, this tumor was not only referred to as red dot basal cell carcinoma, but also regularly encountered [4].
In December 2014, the report of a female with red dot basal cell carcinoma was accepted for publication, and the paper was published in May 2017 [1]. Additional reports of individuals with red dot basal cell carcinoma were published in May 2016 (one female) [3] and March 2017 (three patients: two male and one female) [2]. The latter paper also reviewed the features of this clinical variant of basal cell carcinoma [2].
In September 2018, the mother of one of the authors (P.R.C.) shared her personal experience regarding the diagnosis and management of a basal cell carcinoma on the right side of her upper lip. The paper also included a section in which basal cell carcinoma was discussed by the patient’s dermatologist. In this physician’s perspective section, clinical subtypes of basal cell carcinoma—including red dot basal cell carcinoma—were listed in a table and discussed [6].
In February 2019, a comprehensive review of basal cell carcinoma was published [8, 9]. The authors extensively discussed the clinical and histological subtypes of basal cell carcinoma; however, the paper did not include the less common morphologic variant of basal cell carcinoma: red dot basal cell carcinoma. Therefore, for completeness, the equally important red dot basal cell carcinoma was highlighted in a July 2019 letter to the editor [7].
The current article on red dot basal cell carcinoma adds an additional report of a patient with this variant of basal cell carcinoma. It also provides a comprehensive review of the features of this clinical subtype of red dot basal cell carcinoma.
Epidemiology
To the best of the authors’ knowledge (and including the individual presented in the legends of Figs. 1, 2, and 3 in this manuscript), red dot basal cell carcinoma has been described in eight Caucasian patients: three male and three female (Table 2) [1–4]. The gender was not provided for two patients [4]. Hence, the ratio of male to female was 1:1.
Fig. 1.
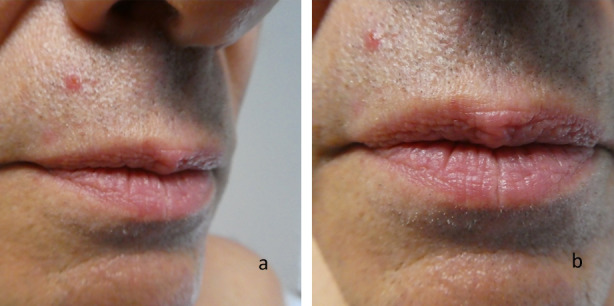
Clinical presentation of a red dot basal cell carcinoma on the right side of the upper lip. A 65-year-old Caucasian male had a history of a junctional dysplastic nevus with moderate atypia on his right distal leg, actinic keratoses, a superficial basal cell carcinoma on his right chest, and a superficial spreading malignant melanoma, Breslow thickness 0.2 mm, on his left anterior thigh. He presented with an asymptomatic lesion of not more than 3 months duration; there had been no trauma to the site. Right (a) and frontal (b) views showed a pink to red-colored 4 × 6 mm papule with a central red dot on the right side of the upper lip. The clinical differential diagnosis included an angioma, a basal cell carcinoma and an adnexal tumor. A shave biopsy was performed
Fig. 2.
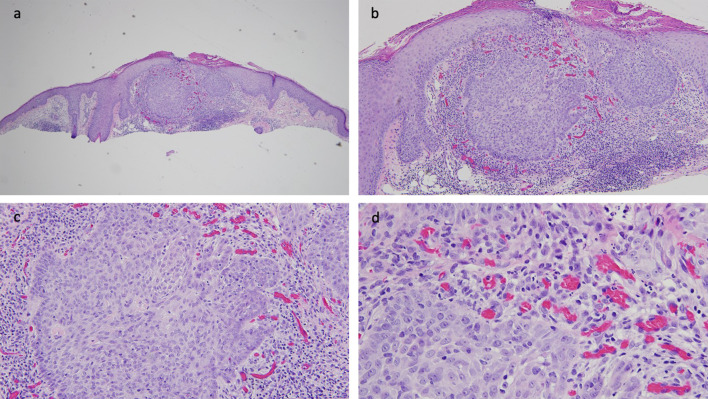
Microscopic features of a red dot basal cell carcinoma that was present on the right side of the upper lip of a 65-year-old male. Distant (a) and closer (b–d) views of the biopsy tissue specimen of the red dot basal cell carcinoma were stained with hematoxylin and eosin stains. There are mounds of parakeratosis overlying an acanthotic epidermis. In the center of the specimen, there are superficial buds and a nodular aggregate of basaloid tumor cells that extend from the overlying epithelium into the underlying dermis. Adjacent and beneath the nests of basal cell carcinoma, there is a dense lymphocytic inflammatory infiltrate. In the papillary dermis, between the epidermis and the tumor, there are numerous benign dilated endothelial-lined vascular spaces that contain erythrocytes (hematoxylin and eosin: a ×4; b ×10; c ×20; d ×40)
Fig. 3.
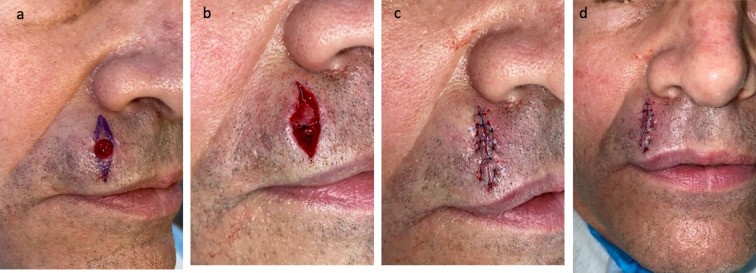
Excision of red dot basal cell carcinoma and closure of the surgical wound on the right side of the upper lip of a 65-year-old male. Correlation of the clinical presentation and the pathologic findings of the lesion on the right side of the upper lip of the 65-year-old male was a red dot basal cell carcinoma (with superficial and nodular tumor aggregates). The tumor was excised using the Mohs technique. Curettage of the site prior to the initial incision was unremarkable. One stage was required for cancer removal. The postoperative defect was 8 × 9 mm; the purple markings show the planned excisions of the standing cones to convert the oval wound into an ellipse (a). The final defect (b) was closed with a complex linear repair using a synthetic absorbable monofilament 5–0 polydioxanone (PDS) suture for closing the dermis and a synthetic nonabsorbable monofilament 6–0 polypropylene (prolene) suture for bring the epidermal edges together; side (c) and frontal (d) views show the 2.8 cm linear closure on the right side of the upper lip after completion of suturing. There has been no recurrence 3 months after surgery
Table 2.
Characteristics of patients with red dot basal cell carcinoma
| C | A G |
History of AK or skin tumor | Site of BCC | Size: Preop Postop Rat (Po:Pr) |
Dias | Pathology subtype of BCC | Treatment | Follow up: Recur Dur |
References |
|---|---|---|---|---|---|---|---|---|---|
| 1 |
50 W |
AK 4 BCC 2 MM 1 SCC |
Left thigh |
3 × 3 NS NS |
NS | Nodular | ED and C |
– NS |
[1] |
| 2 |
65 M |
AK 1 BCC 1 JDN 1 MM |
Right upper lip |
4 × 6 8 × 9 3:1 |
NS | Nodular and superficial |
Mohs, S1 CLR |
– 3 months |
CR |
| 3 |
70 M |
AK 4 BCC |
Nasal tip |
3 × 3 11 × 10 12:1 |
NS | Nodular and superficial |
Mohs, S2 FTSG |
– 5 months |
[2], C1 |
| 4 |
71 M |
AK 1 BCC 1 SCC |
Left nostril |
2 × 2 NS NS |
– | Nodular | Mohs |
– NS |
[2], C2 |
| 5 |
72 W |
AK 2 BCC 1 SCC |
Left nasal bridge |
2 × 3 10 × 10 17:1 |
+ | Superficial |
Mohs, S2 FTSG |
– NS |
[3] |
| 6 |
74 W |
AK 8 BCC |
Left mid back |
7 × 9 15 × 11 3:1 |
+ | Nodular and superficial |
Mohs, S1 CLC |
– 6 mon |
[2], C3 |
| 7 |
NS NS |
NS | Left nasal ala |
2 × 2 4 × 5 5:1 |
NS | NS | Mohs |
NS NS |
[4], C1 |
| 8 |
NS NS |
NS | Right nasal sidewall |
4 × 4 15 × 15 14:1 |
NS | NS | Mohs |
NS NS |
[4], C2 |
A age (in years at diagnosis), AK actinic keratosis, BCC basal cell carcinoma, C case, CLC complex layered closure, CLR complex linear repair, CR current report, Dias diascopy (blanchable after compression of the lesion with a glass microscope slide is positive), Dur duration (months) free of tumor after treatment, ED and C electrodessication and curettage, FTSG full thickness skin graft, G gender, JDN junctional dysplastic nevus with moderate dysplasia, M man, NS not stated, Preop preoperative size of tumor in millimeters, Postop postoperative wound size in millimeters, Rat (Po:Pr) ratio of postoperative tumor area to preoperative wound area, Recur recurrence of basal cell carcinoma, S1 one Mohs stage to clear tumor, S2 two Mohs stages to clear tumor, SCC squamous cell carcinoma, W woman, x by, + present, – absent
The onset age of red dot basal cell carcinoma ranged from 65 to 71 years for male patients (median, 70 years; mean, 69 years). For female patients, the onset age ranged from 50 to 74 years (median, 72 years; mean, 65 years). Overall, the age ranged from 50 to 74 years (median, 71 years; mean, 67 years); the onset age was not provided for two patients [4].
Associated Skin Tumors
All of the red dot basal cell carcinoma patients had either actinic keratoses, dysplastic nevus, and/or skin cancer (Table 2) [1–4]. One male had a junctional dysplastic nevus with moderate atypia on his right distal leg. All of the patients had a history of actinic keratoses.
All of the red dot basal cell carcinoma patients had a history of nonmelanoma skin cancer. They had either one (two patients), two (one patient), four (two patients), or eight (one patient) prior basal cell carcinomas. Three patients also had a prior squamous cell carcinoma (Table 2) [1–4].
Malignant melanoma had occurred in two patients. One male had a superficial spreading malignant melanoma on his left thigh. One female had two primary superficial spreading malignant melanomas on her back (Table 2) [1–4].
Past Medical History
A past medical history for medical conditions was present in four of the patients [2, 3]; the male patient described in this paper was healthy and other medical conditions were not stated for the other three patients [1, 4]. The medical conditions observed included arrythmia [3], benign paroxysmal positional vertigo [2], coronary artery disease [2], diabetes mellitus [2], deep vein thrombosis [2], gout [2], hyperlipidemia [2], hypertension [2], mantle cell lymphoma [2], nephrolithiasis [2], overactive active urinary bladder [3], and obstructive sleep apnea [2]. We suspect that these medical conditions did not have a contributory etiologic role in the development of the patient’s red dot basal cell carcinoma since they do not influence the pathogenesis of other clinical variants of basal cell carcinoma.
Duration
The duration that the red dot basal cell carcinoma was present prior to diagnosis was only described in three of the patients: two females [2, 3] and the 65-year-old male. It ranged from 3 months (in the male patient) to more than 6 months [2]. The median duration was more than 3 months [3], and the mean duration was approximately 4 months.
Symptoms
Red dot basal cell carcinoma typically presents without any tumor-associated symptoms. The 65-year-old male’s upper lip cancer was asymptomatic. In addition, the reports of six of the red dot basal cell carcinoma patients did not mention any symptoms related to the tumor [1, 2, 4].
The 72-year-old female patient initially presented with an asymptomatic, diascopy-blanching, red macule on her distal left nasal bridge. The initial clinical impression was a telangiectasia; a decision was made to check the lesion again in 3 months. During the observation period, she experienced occasional bleeding from the site; examination showed a concurrent flesh-colored papule that had developed beneath the red macule [3].
Location
The red dot basal cell carcinoma commonly occurred on sun-exposed areas [5]. The face (75%, six patients) was the most frequent location for red dot basal cell carcinomas; they were located either on the nose (five patients) or the upper lip (one patient). The red dot basal cell carcinoma occurred on a non-facial site of the other two patients—either on the mid back or the thigh (Table 2) [1–4].
The red dot basal cell carcinoma more often occurred on the left side (five patients) than the right side (two patients). The tumor occurred on the midline of the nasal tip in one male. Whether the side of body on which the tumor occurs is coincidental or related to social and/or work exposure to sun light remains to be determined (Table 2) [1–4].
Clinical Presentation
Red dot basal cell carcinoma is a solitary tumor. Johnson et al. mentioned that the tumor presented as a small, 1–2-mm red papule—sometimes with a halo. In addition, they noted that red dot basal cell carcinoma usually appeared on sites that had been exposed to the sun and that early diagnosis usually required a high index of suspicion [5].
Tromberg et al. also commented that red dot basal cell carcinoma was a small lesion, sometimes only 1 mm in size. They noted that it could present as a red crust or dot vessel [4]. They also mentioned that a subtle shiny induration or pearling of the epidermis around the red dot could be seen when the surrounding skin was stretched [4].
Based on the previously reported patients with red dot basal cell carcinoma, the size of the tumor usually ranged from 2 × 2 mm to 3 × 3 mm. Two of the patients’ tumors were either 4 × 4 mm (on the nasal side wall) or 4 × 6 mm (on the upper lip). However, one patient had a larger tumor (7 × 9 mm) on her mid back (Table 2) [1–4].
The morphology of a red dot basal cell carcinoma was a small red macule or papule. Sometimes, similar to the male with the upper lip tumor, the papule was a lighter shade of red or pink, and the central portion of the papule was bright red (Fig. 1). Another clinical presentation of tumor was an erythema-surrounded red area. In addition, in some patients, such as the male with the nasal tip red dot basal cell carcinoma, the tumor appeared as a flesh-colored papule surrounding the red dot. This presentation gave the lesion the appearance of being surrounded by a skin-colored halo and was suggestive of the halo basal cell carcinoma designation described by Johnson et al. [5].
Diagnosis
A red dot basal cell carcinoma is a malignant epithelial lesion that can clinically masquerade as a benign vascular lesion such as a hemangioma or a telangiectasia. Diascopy and dermoscopy may be utilized in an attempt to establish the diagnosis. However, results of the former are unreliable for diagnosis, and observations of the latter have not been described.
Diascopy is a procedure that uses a clear material to compress a lesion. The tissue becomes blanched in appearance if blood within the lesion is dissipated. Therefore, a vascular lesion—such as a hemangioma or telangiectasia—would be expected to blanch after diascopy [10].
The results of diascopy have not been consistent when evaluating red dot basal cell carcinoma. One tumor on a male patient’s nostril did not blanch [2]. However two female patients’ cancers—on either the nasal bridge or mid back—blanched after being pressed with a glass microscope slide [2, 3]; this observation is not unexpected and likely reflects evacuation of erythrocytes from the proliferation of endothelial-lined vessels in the upper dermis of this basal cell carcinoma variant.
A dermatoscope is an instrument consisting of a magnifier, a (polarized or non-polarized) light source, and a transparent material. Dermoscopy uses a dermatoscope to noninvasively observe the epidermis and papillary dermis. In addition to evaluating pigmented lesions, a dermatoscope can be used not only to examine for infectious organisms (such as mites), but also to assess for nonmelanocytic benign and malignant tumors [11–17].
Dermoscopy of basal cell carcinomas has been well defined; however, a single feature has not been found to uniquely correlate with a specific pathologic basal cell carcinoma subtype. The most common dermoscopic vascular structures were arborizing vessels and short-fine telangiectasias; the former usually occur in infiltrative and nodular basal cell carcinoma, while the latter are present most frequently in superficial basal cell carcinoma. Frequently observed dermoscopic pigmented structures are leaf-like areas and spike wheel areas (both of which are noted predominantly in superficial basal cell carcinoma), and large blue-gray ovoid nests (which are more often seen in nodular basal cell carcinoma than superficial basal cell carcinoma). Finally, shiny white structures (noted in all basal cell carcinoma subtypes) and multiple small erosions (more often seen in superficial basal cell carcinoma than nodular basal cell carcinoma) are nonvascular and nonpigmented dermoscopic structures observed in basal cell carcinoma. To date, the dermatoscopic evaluation of a red dot basal cell carcinoma has not been reported [11–14].
In contrast to basal cell carcinoma, dermoscopy of a hemangioma shows numerous, widespread, round or oval red-blue lacunae. This finding corresponds to the large dilated vascular spaces in the superficial dermis. Clinical or dermoscopic observation of telangiectasia does not exclude the possibility of a basal cell carcinoma since they can be present not only in red dot basal cell carcinoma but also other subtypes of basal cell carcinoma [12, 15–18].
To definitively establish the diagnosis of red dot basal cell carcinoma, microscopic evaluation of the lesion is necessary. An incisional biopsy (using a punch or shave technique) that provides adequate tissue for study can be performed. Alternatively, an excisional biopsy (thereby providing the entire lesion for evaluation) can be executed.
Pathology
The pathology subtypes of red dot basal cell carcinoma, for the patients whose tumors were described in the literature (Table 2) [1–4], were all histologically non-aggressive. They were either nodular and superficial (three patients), nodular (two patients) or superficial (one patient); in addition, Tromberg et al. mentioned that some of the tumors they observed had more macronodular and superficial components [4]. Specifically, there were nodular aggregates or superficial buds or both of basaloid tumor cells extending from the epidermis into the dermis. In addition, in the central portion of the lesion between the overlying epidermis and the underlying neoplasm in the dermis, there were numerous benign-appearing endothelial-lined vessels containing erythrocytes. In some of the lesions, there were also extravasated red blood cells in the upper dermis (Fig. 2).
In Tromberg et al.’s article, they presented images of two patients with red dot basal cell carcinoma—before and after Mohs surgery—and emphasized several salient features about this tumor, but they do not provide the pathology subtype for the patients they included. However, based on their clinical experience, the investigators made two observations: they did not notice any correlation between the red dot basal cell carcinoma clinical subtype and a particular basal cell carcinoma pathology subtype, and some of the red dot basal cell carcinoma were unexpectantly aggressive with an infiltrative basal cell carcinoma pathology subtype consisting of an extensive pattern of invasive strands of tumor cells [4].
Differential Diagnosis
The principal clinical differential diagnosis of a red dot basal cell carcinoma is a benign vascular lesion such as a hemangioma or telangiectasia. Diascopy may not be sufficient to differentiate red dot basal cell carcinoma from these vascular lesions. However, dermoscopy may be able to differentiate a red dot basal cell carcinoma from a hemangioma [1–3].
There are several etiologies for cutaneous telangiectasia. These include part of a genetic condition, a secondary component of an acquired disease, hormonal-related, physical damage, and a primary component of a cutaneous disease. Basal cell carcinoma is a primary skin tumor that can be associated with telangiectasia. Therefore, telangiectasia is not only in the differential diagnosis of red dot basal cell carcinoma, but also can be a component of red dot basal cell carcinoma [1–3, 18].
The clinical differential diagnosis of red facial papules also includes inflammatory conditions such as acne and ruptured follicles. In addition, both benign and malignant adnexal tumors can present with this morphologic appearance. Therefore, a lesion biopsy may be warranted to establish the diagnosis of a new red papule—particularly if it occurs on the face or a sun exposed area.
The pathologic differential diagnosis of a red dot basal cell carcinoma might include benign and malignant tumors comprised of basaloid cells. These would include benign tumors of either follicular derivation (such as basaloid follicular hamartoma, trichoblastoma and trichoepithelioma) or eccrine origin (such as poroma and spiradenoma). In addition they would include malignant neoplasms such as Merkel cell carcinoma.
Pathogenesis
We speculate that the pathogenesis of the red dot basal cell carcinoma is similar to that of other clinical subtypes of basal cell carcinoma. Basal cell carcinoma carcinogenesis is related to activation of the Hedgehog signaling pathway and exposure to ultraviolet B radiation (often from sunlight) is a significant promoter of keratinocyte mutagenesis. In addition, congenital conditions (such as certain genodermatoses) and acquired etiologies (such as ionizing radiation, immunosuppression in organ transplant patients, and the photosensitizing drug psoralen plus ultraviolet A light phototherapy) can also increase the risk of developing basal cell carcinoma [6, 8, 9, 19–21].
It remains to be determined why red dot basal cell carcinoma presents with its unique, albeit peculiar, morphology. Indeed, this clinical variant of basal cell carcinoma is not associated with a specific pathologic subtype. However, the abundance of blood vessels and extravasated red blood cells associated with the tumor may result from neoplasm-induced increased levels of vascular endothelial growth factor localized to the central portion of the carcinoma.
The majority of basal cell carcinoma present as a discrete localized tumor. However, in some individuals, the basal cell carcinoma can be locally advanced or metastatic. In contrast to patients with a discrete localized basal cell carcinoma in whom excision or destructive management or radiotherapy or topical agents may be considered to treat the basal cell carcinoma, individuals with advance or metastatic basal cell carcinoma are likely to require treatment with targeted and immune therapies [19–25].
Treatment
The management of red dot basal cell carcinoma has either been Mohs surgery (seven patients) or electrodessication and curettage (one patient); the latter patient was a 50-year-old female who refused to have her left thigh red dot basal cell carcinoma excised (Table 2) [1–4]. The tumor was cleared after either one (two patients) or two (two patients) Mohs stages; the number of stages was not described for three of the patients. Once the tumor was cleared, the wound was managed with either a full thickness graft (two patients), a complexed layered closure (one patient), or a complex linear repair (one patient) (Fig. 3).
In their letter to the editor, Drs. Tromberg and Dinehart collaborated with Dr. Amonette to share their experience and insight regarding red dot basal cell carcinoma. They express that this clinical variant of basal cell carcinoma is a common phenotype that they observed and treated in their practices. However, evaluation of their patients’ postoperative wound size as compared with that of the preoperative tumor seems to create two categories of biologic behavior for this basal cell carcinoma subtype [4].
Initially Tromberg et al. described the red dot basal cell carcinoma as a distinct and early presentation of basal cell carcinoma; they also mentioned that the tumors—despite having indistinct clinical margins—remain small [4]. Indeed, the ratio of the postoperative wound size as compared with that of the preoperative tumor on a female patient’s nasal ala was only 5:1 [4]. Two other patients—with tumors on either their mid back [2] or upper lip (65-year-old male)—also had a smaller ratio of 3:1 (Table 2) [1–4].
However, as Tromberg et al. also commented occasionally, the observed clinical margins of the red dot basal cell carcinoma failed to provide an accurate depiction of the tumor’s actual size; indeed the tumor’s lateral spread, as discovered during Mohs surgery, was significantly greater than had been expected based on the preoperative size of the lesion [4]. One of their patients, a female with a red dot basal cell carcinoma on her nasal sidewall, had a ratio of the postoperative wound size as compared with that of the preoperative tumor of 14:1 [4]. Two other patients with red dot basal cell carcinoma on either the tip [2] or bridge [3] of their nose also had a larger ratio of either 12:1 [2] or 17:1 [3], respectively.
Prognosis
Information from follow-up examination was provided for only six patients with red dot basal cell carcinoma (Table 2) [1–4]. None of the patients had recurrence of their tumor. The duration of cancer-free follow-up, provided for three patients, ranged from 3 to 6 months (median and mean, 5 months).
Conclusions
Red dot basal cell carcinoma is a distinctive clinical subtype of basal cell carcinoma. Although mentioned earlier in literature, to the best of the authors’ knowledge, patients with this basal cell carcinoma variant were initially described in 2012. To date, red dot basal cell carcinoma has only been reported in eight patients: three male, three female, and two individuals whose gender was not provided. Age at diagnosis ranged from 50 to 74 years (median 71 years, mean 67 years). All of the red dot basal cell carcinoma patients had prior history of actinic keratoses and basal cell carcinoma. Some patients had a prior squamous cell carcinoma (three patients), a malignant melanoma (two patients), or a dysplastic nevus (one patient). Four had other medical conditions; however, these probably did not contribute to the development of their red dot basal cell carcinoma. The tumor was usually asymptomatic and appeared within the previous 3–6 months; one female patient’s cancer occasionally bled. It was frequently located on the sun-exposed areas of the face: the nose in five patients, and the upper lip in one patient. The other tumors were on the mid back or thigh. The red dot basal cell carcinoma was a small solitary lesion; most of the tumors were 4 mm or less in diameter; one female patient’s lesion was 9 mm. It typically presented as a red macule or papule; however, it sometimes appeared as a flesh-colored or pink to light-red papule with a bright-red central area. The diagnosis for all of the red dot basal cell carcinoma were biopsy-confirmed. Microscopic features showed nodular aggregates or superficial buds or both of basaloid tumor cells extending from the epidermis into the dermis with numerous benign-appearing endothelial-lined vessels containing erythrocytes in the central portion of the lesion between the overlying epidermis and the underlying neoplasm. The pathology subtype of basal cell carcinoma was either nodular and superficial (three patients), nodular (two patients), or superficial (one patient). The clinical differential diagnosis of red dot basal cell carcinoma includes not only benign vascular lesions (such as hemangioma and telangiectasia), but also inflammatory conditions (such as acne and ruptured follicles), and benign or malignant adnexal tumors. Benign and malignant basaloid cell neoplasms are in the pathologic differential diagnosis of red dot basal cell carcinoma. The pathogenesis of red dot basal cell carcinoma is similar to that of other clinical subtypes of basal cell carcinoma. Mohs surgery (requiring one or two stages to clear the tumor) was used to treat seven of the red dot basal cell carcinomas. Based on the ratio of the postoperative wound size as compared with that of the preoperative tumor, red dot basal cell carcinoma has two categories of biologic behavior; the ratio was 5:1 or less for three patients, and greater than 12:1 for three patients. There was no recurrence of the red dot basal cell carcinoma after treatment; however, follow-up (only for 3–6 months) was only provided for six of the patients. In conclusion, red dot basal cell carcinoma is a unique morphologic variant of basal cell carcinoma which may be more prevalent than suggested by the number of reported patients with this subtype of basal cell carcinoma.
Acknowledgements
We thank the participant of the study.
Funding
No funding or sponsorship was received for publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval of the version to be published. The opinions expressed in the manuscript are those of the authors.
Disclosures
Marta Torres-Quiñones and Nathan S. Uelbelhoer have nothing to disclose. Philip R. Cohen Dr. is a consultant for ParaPRO, and is a member of the journal’s Editorial Board.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain studies with human participants or animals performed by any of the authors. However, the patient signed a consent providing permission for including the clinical photographs in this article.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1.Cohen PR. Red dot basal cell carcinoma. J Clin Aesthet Dermatol. 2017;10(5):56–58. [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen PR. Red dot basal cell carcinoma: report of cases and review of this unique presentation of basal cell carcinoma. Cureus. 2017;9(3):e1110. doi: 10.7759/cureus.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loh TY, Cohen PR. Red dot basal cell carcinoma: an unusual variant of a common malignancy. J Drugs Dermatol. 2016;15(5):645–647. [PubMed] [Google Scholar]
- 4.Tromberg J, Dinehart S, Amonette R. “Red dot” basal cell carcinoma: an early and distinct clinical presentation. Dermatol Surg. 2012;38(10):1761–1763. doi: 10.1111/j.1524-4725.2012.02539.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson TM, Tschen J, Ho C, Lowe L, Nelson BR. Unusual basal cell carcinomas. Cutis. 1994;54(2):85–92. [PubMed] [Google Scholar]
- 6.Cohen BJ, Cohen ES, Cohen PR. Basal cell carcinoma: a patient and physician’s experience. Dermatol Ther (Heidelb) 2018;8(3):329–337. doi: 10.1007/s13555-018-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen PR. Basal cell carcinoma: additional subtypes and therapeutic advances. J Am Acad Dermatol. 2019;81(1):e17. doi: 10.1016/j.jaad.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 8.Cameron MC, Lee E, Hibler BP, Barker CA, Mori S, Cordova M, Nehal KS, Rossi AM. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303–317. doi: 10.1016/j.jaad.2018.03.060. [DOI] [PubMed] [Google Scholar]
- 9.Cameron MC, Lee E, Hibler BP, Giordano CN, Barker CA, Mori S, Cordova M, Nehal KS, Rossi AM. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80(2):321–339. doi: 10.1016/j.jaad.2018.02.083. [DOI] [PubMed] [Google Scholar]
- 10.Nadeau C, Stoopler ET. The clinical value of diascopy. J Can Dent Assoc. 2013;79:d11. [PubMed] [Google Scholar]
- 11.Fargnoli MC, Kostaki D, Piccioni A, Micantonio T, Peris K. Dermoscopy in the diagnosis and management of non-melanoma skin cancers. Eur J Dermatol. 2012;22(4):456–463. doi: 10.1684/ejd.2012.1727. [DOI] [PubMed] [Google Scholar]
- 12.Ayhan E, Ucmak D, Akkurt ZM. Vascular structures in dermoscopy. An Bras Dermatol. 2015;90(4):545–553. doi: 10.1590/abd1806-4841.20153452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiter O, Mimouni I, Dusza S, Halpern AC, Leshern YA, Marghoob AA. Dermoscopy features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol. 2019 doi: 10.1016/j.js.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Salafranca M, Ara M, Zaballos P. Dermoscopy in basal cell carcinoma: an updated review. Actas Dermosifiliogr. 2020 doi: 10.1016/j.ad.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2015;88(7):441–450. [PubMed] [Google Scholar]
- 16.Martin JM, Bella-Navarro R, Jorda E. Vascular patterns in dermoscopy. Actas Dermosifiliogr. 2012;103(5):357–375. doi: 10.1016/j.ad.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Usatine RP, Shama LK, Marghoob AA, James N. Dermoscopy in family medicine: a primer. J Fam Pract. 2018;67(12):E1–E11. [PubMed] [Google Scholar]
- 18.Goldman MP, Bennett RG. Treatment of telangiectasia: a review. J Am Acad Dermatol. 1987;17:1667–2182. doi: 10.1016/S0190-9622(87)70187-X. [DOI] [PubMed] [Google Scholar]
- 19.Nikanjam M, Cohen PR, Kato S, Sicklick JK, Kurzrock R. Advanced basal cell cancer: concise review of molecular characteristics and novel targeted and immune therapeutics. Ann Oncol. 2018;29(11):2192–2199. doi: 10.1093/annonc/mdy412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tampa M, Georgescu SR, Mitran CI, Mitran MI, Matei C, Scheau C, Constantin C, Neagu M. Recent advances in signaling pathways comprehension as carcinogenesis triggers in bssal cell carcinoma. J Clin Med. 2020;9(9):E3010. doi: 10.3390/jcm9093010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman AM, Kato S, Cohen PR, Boichard A, Frampton G, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Genomic landscape of advanced basal cell carcinoma: implications for precision treatment with targeted and immune therapies. Oncoimmunology. 2017;7(3):e1404217. doi: 10.1080/2162402X.2017.1404217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen PR, Kato S, Goodman AM, Ikeda S, Kurzrock R. Appearance of new cutaneous superficial basal cell carcinomas during successful nivolumab treatment of refractory metastatic disease: implications for immunotherapy in early versus late disease. Int J Mol Sci. 2017;18(8):1663. doi: 10.3390/ijms18081663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda S, Goodman AM, Cohen PR, Jensen TJ, Ellison CK, Frampton G, Miller V, Patel SP, Kurzrock R. Metastatic basal cell carcinoma with amplification of PD-L1: exceptional response to anti-PD1 therapy. NPJ Genom Med. 2016;1:16037. doi: 10.1038/npjgenmed.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen PR, Kurzrock R. Basal cell carcinoma: management of advanced or metastatic cancer with checkpoint inhibitors and concurrent paradoxical development of new superficial tumors. J Am Acad Dermatol. 2020;82(6):e253–e254. doi: 10.1016/j.jaad.2020.02.052. [DOI] [PubMed] [Google Scholar]
- 25.Cohen PR, Kurzrock R. Reply to “Effective therapy for advanced basal cell carcinoma”. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.06.988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.