Figure 4. Contact-dependent growth inhibition (CDI).
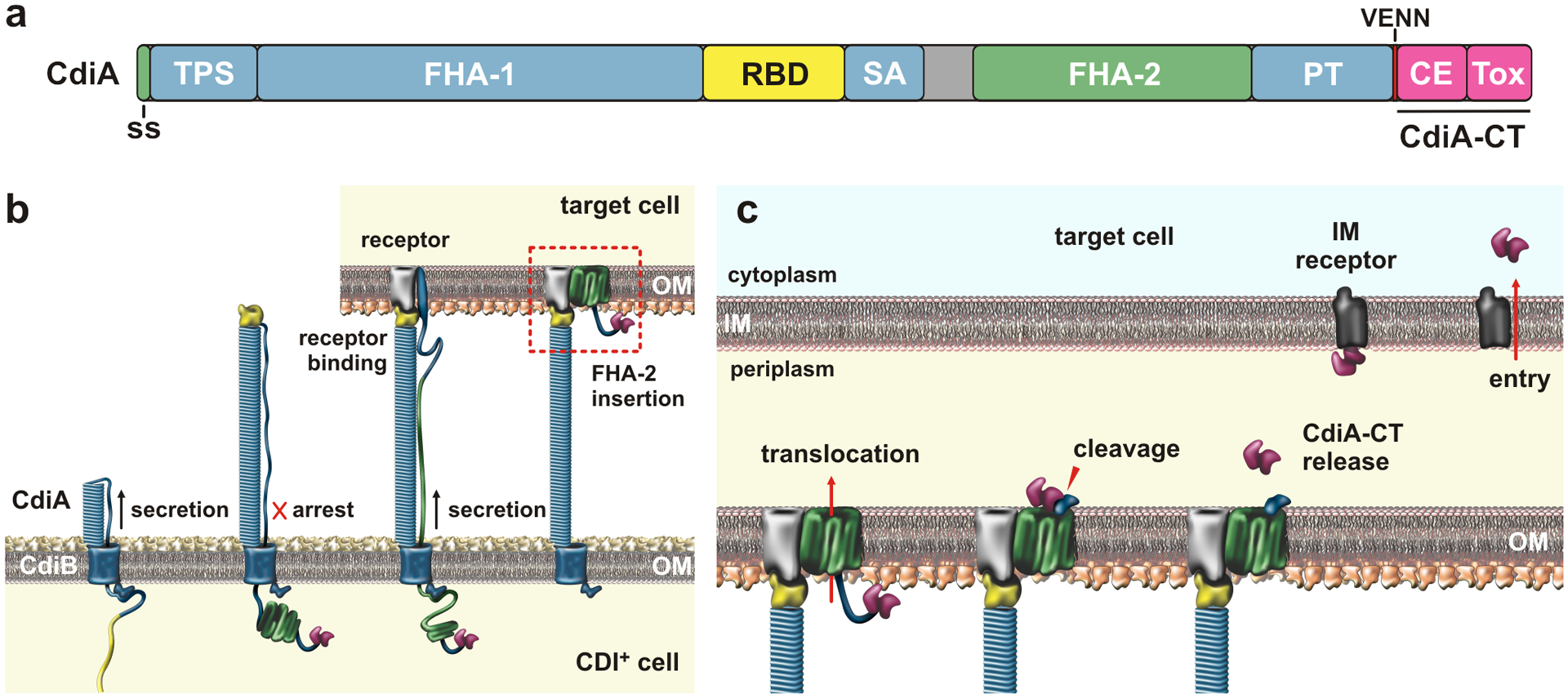
a) CdiA domain architecture. The CdiA-CT is composed of cytoplasm entry (CE) and toxin domains; Abbreviations: ss, signal sequence; TPS, two-partner secretion transport domain; RBD, receptor-binding domain; SA, secretion arrest domain; PT, pretoxin domain; and CE, cytoplasm entry domain. b) CdiA biogenesis. CdiB guides CdiA export across the outer membrane (OM). The C-terminal half of CdiA remains sequestered in the periplasm due to a programmed secretion arrest. CdiA export resumes upon binding receptor, and the FHA-2 domain integrates into the target-cell OM to form a toxin translocation conduit. c) Toxin delivery. Once transferred into the periplasm, the CdiA-CT is released for a second translocation step across the inner membrane (IM). The final step is mediated by cytoplasm entry domains, which interact with membrane proteins and translocate the C-terminal toxin domain in a pmf-dependent manner. Abbreviations: CE, cytoplasm entry domain; CT, C-terminal region; IM, inner membrane; OM, outer membrane; PT, pretoxin domain; RBD, receptor-binding domain; SA, secretion arrest domain; ss, signal sequence; Tox, toxin domain; TPS, two-partner secretion transport domain.
