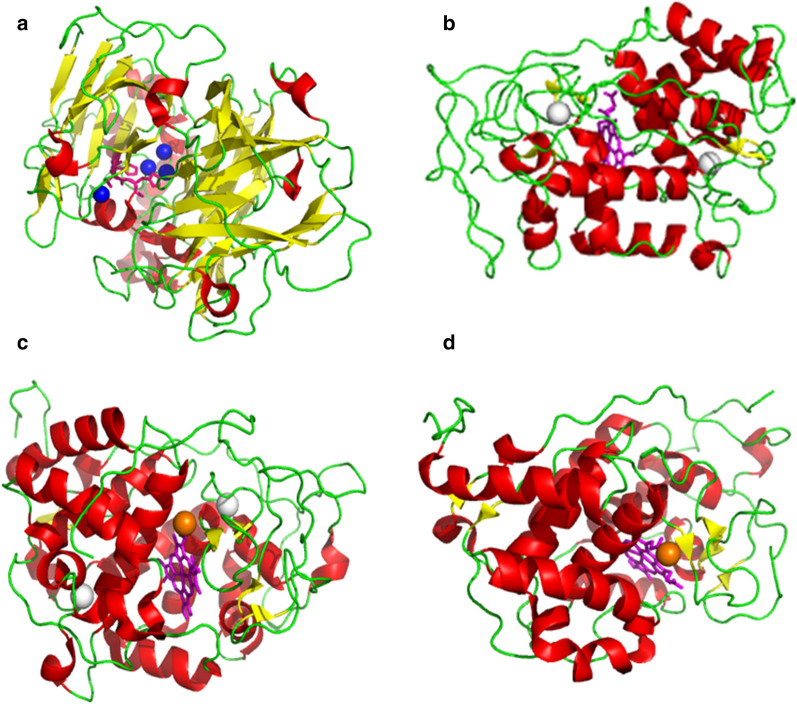
Fig. 2.
Structures of ligninolytic enzymes. The red, yellow, and green colored regions represent α-helix, β-sheet, and random coil, respectively. a Laccase (PDB ID: 1GYC) from Trametes versicolor [78] has a well-conserved active site with four copper, and T1 copper is connected to the trinuclear cluster by a His-Cys-His tripeptide. b Lip (PDB ID: 1LGA) of Phanerochaete chrysosporium [85] contains two calcium ions, four disulfide bonds, and a heme-containing one iron atom as its active site. c MnP (PDB ID: 1YYD) from P. chrysosporium [89] shows the active sites of Glu35, Glu39, and Asp179 residues as well as the Mn2+ ion. d VP (PDB ID: 2BOQ) of Pleurotus eryngii [94] exhibits an Mn2+-binding site and an external Trp residue. The electron transfer pathway towards heme is obtained directly from Mn2+ or relatively long range from Trp