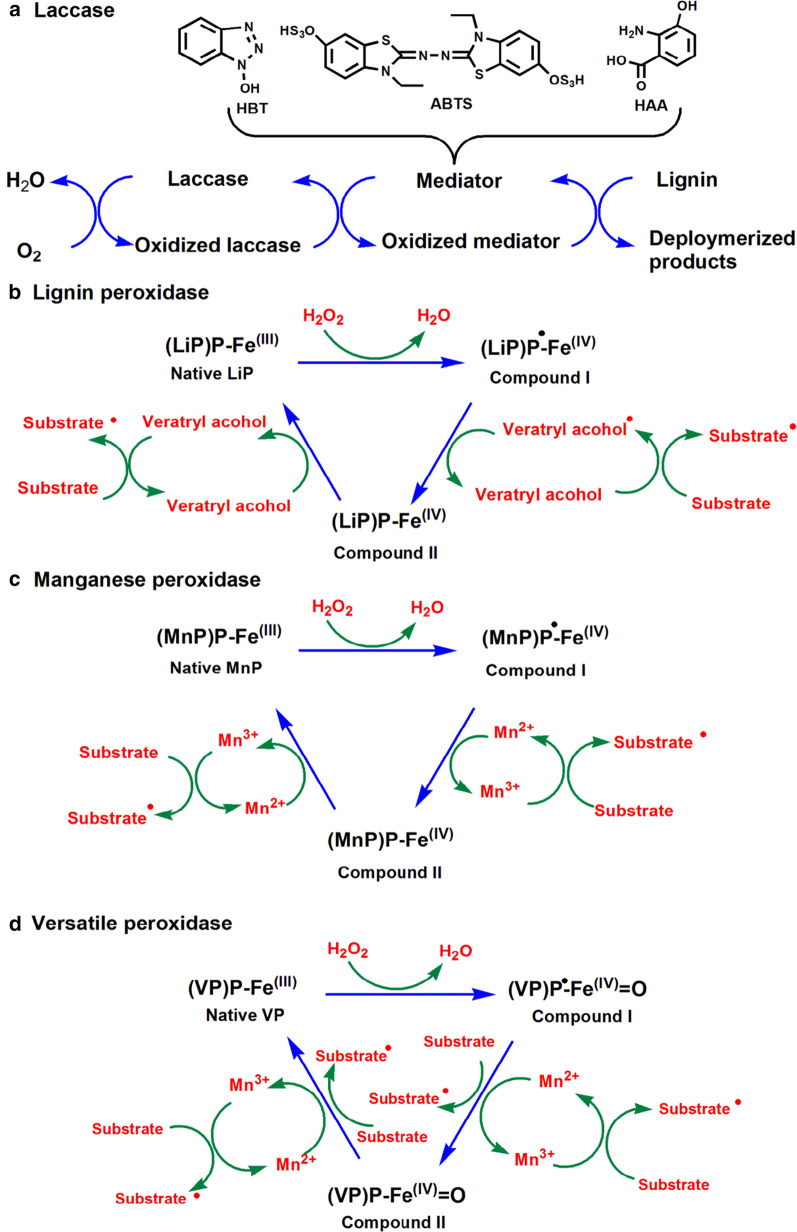
Fig. 3.
Catalytic mechanism of ligninolytic enzymes mediated lignin degradation. a Laccases not only directly oxidize phenolic compounds, but also degrade non-phenolic substrates of lignin in the presence of chemical mediators [79]. Molecular oxygen is reduced into water. b LiP indirectly degrades lignin via oxidizing veratryl alcohol to the corresponding diffusible cation radical as a direct oxidant on lignin. Two electrons of the native ferric enzyme are oxidized by H2O2 to form compound one, which receives one electron to form compound two. Finally, compound two is returned to the resting native ferric state by gaining one more electron from the reducing substrate [86, 87]. c MnP oxidizes the one-electron donor Mn2+ to Mn3+, which in turn oxidizes a large number of phenolic substrates. The native ferric enzyme initially reacts with H2O2 to form compound one, and an Mn2+ ion donates one electron to the porphyrin intermediate to form compound two. The native enzyme is similarly produced from compound two by obtaining one electron from Mn2+ [90, 91]. d The basic catalytic cycle of VP is similar to the MnP and LiP with the two intermediary compounds one and two