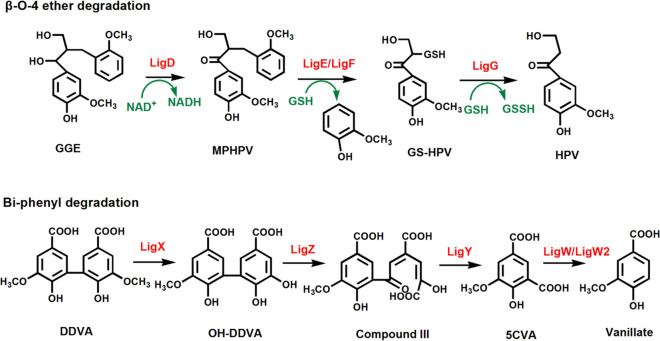
Fig. 4.
Mechanisms of β-O-4 ether and biphenyl linkage degradation. The enzymes involved in the breakdown of β-O-4 aryl ether and biphenyl bonds in bacteria are identified and characterized. In β-O-4 ether degradation, guaiacylglycerol-β-guaiacylethe (GGE) is first degraded to α-(2-methoxyphenoxy)-β-hydroxypropiovanillone (MPHPV), which is then converted to α-glutathionyl-HPV (GS-HPV) and β-hydroxyproppiovanillone (HPV) by LigD (Cα-dehydrogenase), LigE/LigF (β-etherase), and LigG (glutathione lyase) [102]. In biphenyl degradation, 5, 5′-dehydrodivanillate (DDVA) is initially O-demethylated to form 2,2′-3-trihydroxy-3′-methoxy-5,5′-dicarboxybiphenyl (OH-DDVA) by LigX (non-heme iron-dependent demethylase enzyme). The generated substrate is oxidated and cleaved by LigZ (extradiol dioxygenase) and LigY (C–C hydrolase) to produce 5-carboxyvanillic acid (5CVA). Eventually, 5CVA is transformed into the central intermediate vanillic acid by LigW and LigW2 (two decarboxylase enzymes) [106, 108]