Abstract
Osteoporosis is a long-term consequence of spinal cord injury (SCI) that leads to a high risk of fragility fractures. The fracture rate in people with SCI is twice that of the general population. At least 50% of these fractures are associated with clinical complications such as infections. This review article presents key features of osteoporosis after SCI, starting with its aetiology, a description of temporal and spatial changes in the long bones and the subsequent fragility fractures. It then describes the physical and pharmacological approaches that have been used to attenuate the bone loss. Bone loss after SCI has been found to be highly site-specific and characterised by large inter-variability and site-specific changes. The assessment of the available interventions is limited by the quality of the studies and the lack of information on their effect on fractures, but this evaluation suggests that current approaches do not appear to be effective. More studies are required to identify factors influencing rate and magnitude of bone loss following SCI. In addition, it is important to test these interventions at the sites that are most prone to fracture, using detailed imaging techniques, and to associate bone changes with fracture risk. In summary, bone loss following SCI presents a substantial clinical problem. Identification of at-risk individuals and development of more effective interventions are urgently required to reduce this burden.
Keywords: Bisphosphonates, BMD, Disuse Osteoporosis, Electrical Stimulation, Spinal Cord Injury
Introduction
Spinal cord injury (SCI) is a life changing event that has a substantial impact on the individual’s physical and mental health. A global annual incidence of 8.0 to 246.0 cases per million inhabitants has been reported[1] with an increase in the percentage of cases of tetraplegia and complete lesions over a 20 years period (1994-2013)[2,3]. People with SCI experience secondary medical complications such as those affecting their body composition[4,5]. They experience extensive declines in bone density and strength that put them at high risk of fragility fractures and associated morbidity and mortality[6].
This review article summarises different aspects of bone loss and osteoporosis after SCI. It discusses the factors that have been shown to contribute to the SCI-induced bone loss and describes in detail how bone loss develops in the acute and chronic phases of the injury until it reaches its steady state. Factors influencing the large inter-site and inter-individual patterns of bone loss observed in individuals with SCI are also described, in addition to clinical consequences e.g. fractures from SCI-related bone loss and associated complications. It also reviews different physical and pharmacological interventions that have been tested in patients with SCI, to investigate their effectiveness in reversing or attenuating bone loss in acute and chronic phases respectively.
Aetiology of bone loss after SCI
The type of osteoporosis that develops after spinal cord injury (SCI) has been reported to be induced by a combination of factors. The main causal factor is understood to be from mechanical unloading[7], that has also been evidenced by the reported bone loss following space flights and bed rest[8,9]. In addition, neuronal and hormonal changes have been found to contribute to its pathogenesis[7].
Unloading and the bone formation-resorption imbalance
SCI causes immediate disuse and a subsequent loss of biomechanical stress on bones, which is a substantial stimulus for the bone remodelling process controlled by osteocytes[7,8,10]. This absence of mechanical loading leads to an adaptive response involving inhibition of osteoblastic bone formation and increases in osteoclastic bone resorption resulting in demineralisation. In some cases, the imbalance between bone formation and resorption is so great and sustained that it leads to severe bone loss.
This has been widely documented in both acute and chronic SCI. Bone resorption biochemical markers in blood and urine, such as total deoxypyridinoline (DPD) ,N-telopeptide (NTx), serum and urinary type I collagen C-telopeptide (CTx) and hydroxyproline have been found to be significantly increased in acute and chronic SCI[11-14], but significantly lower in chronic than in acute SCI[14]. Nonetheless, elevated levels of DPD were evident in 30% of patients 10 years or more after injury[15]. This significant rise in bone resorption rate after SCI has been found to be associated with a slight increase in osteoblastic bone formation activity, evidenced by minor increases in serum osteocalcin and total alkaline phosphatase[11]. However, there is no consensus on the significance of these small increases in osteoblastic activity, which were found to be minor in some studies[11] but substantial in others[12,16].
The increase in bone resorption and the associated calcium efflux from bones after SCI leads to abnormally high concentrations of calcium in blood (hypercalcemia)[17] and urine (hypercalciuria)[18,19].
Neurovascular changes
Bone also undergoes neurovascular changes caused by the neurological lesion and the subsequent disturbance of bone tissue innervation[7]. Whilst it is clear that the loss of motor function and the associated reduction in bone loading caused by neural damage contribute substantially to bone loss following SCI, the contribution of other neurally-mediated mechanisms of bone loss is less clear[20]. Some of the neuronal changes are directly caused by the significant reduction in sensory and autonomic nerve fibres and nerve-derived factors (neuropeptides) which have been documented to regulate and modulate bone metabolism[7,21,22].
The autonomic nervous system has been documented to regulate skeletal metabolism through different pathways[23,24]. Increased activity of the sympathetic nervous system is known to suppress bone formation and favour bone resorption[23]. Subsequently, one would expect that the attenuated sympathetic activity after SCI should increase bone mass instead. It is clear that the extensive bone loss that occurs after SCI cannot be explained directly by the attenuated sympathetic activity[25]. Furthermore, interruption to the sympathetic system and the subsequent vasomotor irregularity can better (and in part) explain the loss in bone mass[26]. The interrupted sympathetic nerves (which have their processes distributed along bone vessels[27]), lead to vascular modification in the sub-lesional areas[7,28]. Changes in bone blood flow after SCI have been evidenced by high intramedullary pressure and arteriovenous shunting in the legs leading to venous stasis and adverse consequences for bone metabolism[16,29]. The effect of the reduced parasympathetic activity after SCI[30] is an area of active research, and our understanding of its role in regulating bone metabolism is currently limited[23,25], which further emphasises the multi-factorial and complex aetiology of bone loss after SCI.
Hormonal changes
Hypercalciuria is prevalent in the acute phase of SCI as a result of the abnormally high ionised calcium levels which are found to get back to normal during the chronic phase[31]. These changes (in the acute phase) are followed by changes in calcium regulatory hormone levels. Serum intact parathyroid hormone (iPTH) level was found to be suppressed in acute and sub-acute SCI as expected for this negative feedback loop (1-4 months)[11-13,32]. It increases in the chronic phase compared to the acute phase but it stays within or below the lower reference ranges[31]. Only one study reported a decreased level of PTH in the chronic phase, which was associated with normal ionised calcium levels[18]. This decline in PTH has been suggested in this study to be driven by “low-grade increased calcium release”, which in turn indicates persistently elevated bone resorption even after years of injury[18], a hypothesis that is also supported by the high levels of bone resorption markers reported during the chronic phase[31].
Reported changes in vitamin D levels in acute SCI, show reduced levels of 1,25(OH)2 vitamin D (the biologically active form of vitamin D), as a result of bone resorption and the suppression of PTH[13,32]. However, different results were reported for 25-hydroxyvitamin D (25(OH)D) in the acute phase: with one study reporting normal[32], and others reporting low levels[33,34]. Using different reference values to define normal levels of vitamin D level and other factors such as ethnicity and season might have contributed to this variability in their results. In people with chronic SCI, low levels of 25-hydroxyvitamin D (25(OH)D) have been found to be prevalent[34-36], which might be accompanied by mild secondary hyperthyroidism[37]. This could be due to limited exposure to sunlight, prescription of medications that increase vitamin D metabolism and, perhaps, restricted dairy intake[36]. Only one study reported normal levels of 25(OH)D in the chronic phase, which was explained by the majority of medically stable and active participants included in this study[18].
SCI is associated with severe muscle atrophy affecting the sublesional areas[38,39] within the first few days after injury[40,41]. About one third reduction in thigh muscle cross sectional area (CSA) has been found to occur within only 6 to 24 weeks postinjury[40,42]. The denervation and atrophy of these muscles (alongside other factors such as reduced physical activity) are believed to be one of the determinants of insulin resistance[5], which is also associated with increased intra-muscular fat in people with SCI[43].
Sex hormones also play a major role in regulating bone metabolism. Oestrogen has been shown to prevent osteocyte apoptosis[44], and both oestrogen and androgen inhibit bone resorption and promote bone formation via many mechanisms[45,46]. SCI causes the inhibition of sex hormone production and secretion[7]. In people with acute SCI, testosterone levels were significantly lower than in the uninjured control group, with no further change after week 16 post injury[13].
When investigating hypothalamus-pituitary-ovary and hypothalamus-pituitary-thyroid axes in women with SCI, approximately 80% women were found to have at least one axis abnormality[47]. These findings suggest that bone loss can be linked to impaired endocrine function in people with SCI.
Structural and geometric changes in bone after SCI
Densitometric assessment of bone parameters after SCI
Most of the studies on bone loss in the early acute phase and throughout the chronic phase following SCI, have used medical imaging technologies such as dual-energy x-ray absorptiometry (DXA) and peripheral quantitative computed tomography (pQCT).
DXA enables scanning of sites that are generally the most susceptible to osteoporotic fractures in the general population such as the vertebrae and proximal femur (hips) and wrist[48], aiding in detection of osteoporosis and reducing the risk of such clinically and economically costly fractures[49].
Furthermore, its low radiation dose, low price, wide availability and ease of use have all made it the predominantly used technique to diagnose osteoporosis[49,50] and assess susceptibility to fractures clinically[51]. For similar reasons, it has been the default densitometric technique for measuring bone density in clinical trials[50] to evaluate the effectiveness of interventions.
However, DXA measures areal BMD, which is highly affected by bone size, leading to larger bones appearing to have a greater density than smaller bones[49] resulting in both bone loss and fracture risk being underestimated[52]. DXA’s inability to extract three-dimensional measurements of bone geometry limits its utility in describing detailed components of bone structure relevant to fracture risk[49,53]. Moreover, DXA does not distinguish between trabecular and cortical compartments[49]. This limitation of DXA is particularly relevant in disuse osteoporosis due to the differences in the extent and time-course of bone loss in these two bone compartments.
There is good evidence that, in people with SCI, trabecular bone has a more rapid response to disuse[15,54-56] and interventions[51] compared to cortical bone (when considering the percentage of the loss in bone). Therefore, it is recommended to obtain separate, detailed trabecular and cortical BMD measurements in order to assess the effectiveness of different bone interventions.
pQCT and high resolution pQCT (HR-pQCT) provide detailed volumetric parameters of trabecular and cortical compartments[57] making these techniques clinically relevant for this population. They assess volumetric density (vBMD) that considers bone depth (BMC/cm3)[51] and quantify both hard and soft tissues within the region of interest[53]. pQCT also allows detailed, site-specific examination of regions such as the distal femur and proximal tibia[58]. These areas are particularly prone to fracture in individuals with SCI, who lose bone mass exclusively in sublesional sites[56] as shown in Figure 1. In contrast, the most commonly performed regional DXA scans focus on the proximal femur and the lumbar spine.
Figure 1.
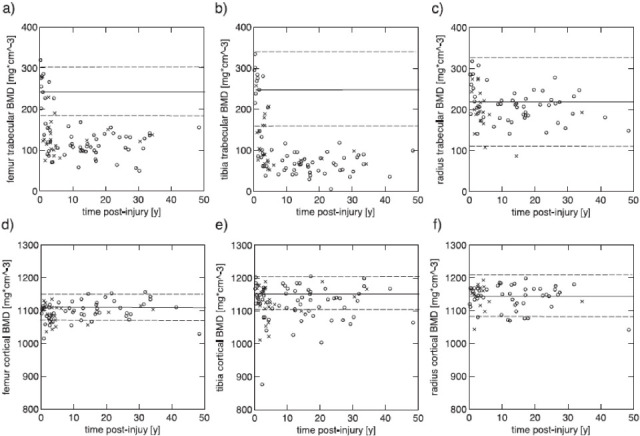
Differences in trabecular (upper row) and cortical (lower row) BMD with time (2 months to 50 years) postinjury in the femur (a,d), tibia (b,e) and radial (c,f) bones within a group of individuals with SCI-induced paraplegia ( 0 ) and tetraplegia (x)[55]. (Reproduced with permission).
HR-pQCT acquires 3D images of bone microarchitecture which is of importance in assessing bone strength in individuals who are susceptible to fractures[49,58], especially people with SCI[57]. It provides trabecular and cortical parameters such as trabecular number, separation, anisotropy and trabecular and cortical thickness[49], shown to improve fracture risk prediction[59,60].
Despite their capabilities, these techniques impose certain practical constraints as imaging of patients with bilateral metal implants or spasticity that could cause movement artefacts is challenging[61]. HR-pQCT can only be used to scan peripheral sites such as distal radius and tibia due to its small field of view, which is compromised to achieve the optimum resolution[58]. Moreover, the 140 mm gantry diameter makes it unusable for scanning obese patients or regions such as the thigh and proximal femur[61]. Nevertheless, these techniques offer detailed bone microarchitecture measurements for people with SCI, who are of higher risk of sustaining osteoporotic fractures mostly in their lower extremities, compared to the spine and upper extremities that are less prone to fracture[57,62]. Bone loss after SCI occurs at sublesional sites only, leading to upper limb bone health being preserved in people with paraplegia whilst people with tetraplegia are at risk of developing osteoporosis in both the upper and lower limbs[63,64].
Notwithstanding the substantial advantages that pQCT and HR-pQCT offer over DXA, they are not currently recommended to be used routinely by clinicians in the diagnosis of osteoporosis, fracture risk prediction or assessment of bone conditions treatments. This is due to the lack of international standards that regulate its clinical use with regards to aspects such as scanning and analysis protocols and anatomical sites, which were recommended recently by Cervinka et al[65].
The absence of standards for the clinical use of pQCT, combined with the ready availability of DXA machines in clinics has driven the development of protocols to make DXA scans more clinically relevant to people with SCI. Different DXA protocols and software have been developed and validated to acquire and analyse images at the knee region, which until now has not been a standard measurement site in DXA. Methods that extract sub-regions from total body DXA scan are not recommended due to their poor repeatability and image resolution[66]. A commercially available GE lunar software for the knee region has FDA approval and is being used for both clinical and research purposes[67-69]. Few studies investigated the accuracy of conventional DXA software designed for lumbar spine, proximal femur and forearm, in predicting BMD and fracture risk at the knee[70,71]. A DXA forearm software has been validated by McPherson et al. (2014) to be used to measure BMD at the knee in people with SCI[72]. It was found to be accurate in short-term assessments and has been proposed as a reliable method to assess BMD at the distal femur and proximal tibia using DXA in clinical trials[71]. The International Society of Clinical Densitometry recommended a BMD analysis protocol based on lumbar spine software for the calculation of BMD at the knee[66,73], until knee-specific software is developed. There remains the need for valid manufacturer-adopted knee software to accurately measure BMD at the distal femur and proximal tibia for clinical use[66].
Time course of bone changes after SCI
Many longitudinal studies on people with SCI emphasise the substantial effect of time since injury on bone structural and geometric parameters during both the acute (5 weeks-12 months)[74] and the chronic phases[75]. Bone resorption markers have been shown to increase significantly to maximum levels within 10-16 weeks postinjury[11]. In the same study, BMD losses in the lower limbs were detected at follow-up (24th week postinjury)[11]. Bone mass continues to decrease with time throughout the first 8 months[76], 12 months[74], 2 years[77] and even throughout the chronic phase (up to 19-25 years)[78,79] but at a slower rate in the later phases compared to the rapid loss during acute phase[54]. However, some studies found that bone loss reaches steady-state phase at 3-8 years postinjury depending on the bone parameter measured[55,75] with about 50% and 60% loss in bone mass in the femoral and tibial epiphyses, and 35% and 25% in the femoral and tibial shafts respectively[55]. Determining the time course of the adaptive modifications in bone geometry and structure is of clinical importance to assess the effectiveness of different rehabilitation interventions in reversing bone loss[75].
Patterns and time course of loss in cortical and trabecular compartments
Bone loss at epiphyseal sites (which are rich in trabecular bone) has been attributed to the decline in trabecular BMD with comparatively little loss from the outer cortical shell[80]. In contrast, bone loss at diaphyseal sites composed primarily of cortical bone is suggested to be characterised by a reduction in wall thickness via endocortical resorption in addition to smaller decreases in cortical BMD[15,53,81] as shown in Figure 2.
Figure 2.
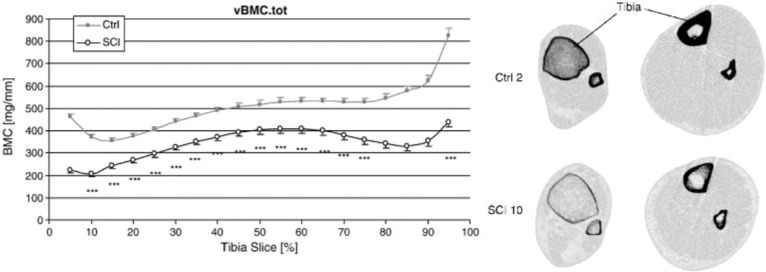
(Left): compares volumetric BMC at different sites along the tibia (starting from distal tibia at 5% of the tibial length and moving toward the proximal epiphysis in steps of 5% (up to 95% of the tibia length) between controls and participants with SCI. It also shows the more pronounced bone loss at the epiphyses compared to the diaphysis between the two groups. (Right): shows pQCT images of the tibia distal epiphysis (left column) and diaphysis (right column) in an uninjured control (upper row) and an individual with SCI (lower row). The decrease in trabecular BMD and the cortical thinning at epiphysis can be seen clearly, alongside the cortical loss/trabecularisation in the diaphysis[80]. (Reproduced with permission).
Exponential decreases in bone parameters with time postinjury (2 months to 50 years) have been described using pQCT, specifically in bone mass, total and trabecular BMD (BMDtot and BMDtrab, respectively) of the femoral and tibia epiphyses, as well as bone mass and cortical CSA of the diaphysis as shown in Figure 1[55]. In a longitudinal study using pQCT scans, significant decline in cortical BMD and cross sectional area (CSA) has been reported alongside the epiphyseal changes within the first 12 months postinjury[74]. In the acute phase, tibial and femoral cortical BMD losses make a substantial contribution to bone loss at these diaphyseal sites[74]. It has been suggested that acute cortical BMD losses may be transient, resulting from increased remodelling in the early stages post-injury[55]. Indeed, in the chronic phase cortical bone losses at these sites appear primarily attributable to decreased cortical bone cross-sectional area with little or no contribution of BMD losses reported[55]. Nevertheless, the loss in trabecular BMD seems to occur at a more rapid rate acutely and is of greater magnitude in the chronic phase than that in the cortical bone[55,82] as shown in Figure 1. Lower BMD in the proximal femur[6,79,83], femoral shaft and lumbar spine[83] have also been correlated with time since injury.
Characteristic temporal and site-specific patterns
Acute SCI
During acute SCI (measured between week 8 and 12 months postinjury), DXA reveals significantly lower BMD in the lower limbs[11,13], total body, pelvis[13], proximal femur[14], midshaft and distal femur[84] with no difference in the hip (between week 8 and week 24 postinjury[11], although BMD loss might have been detected sooner than 24 weeks if the protocol had allowed it), lumbar spine or radius[11,13,14]. After only one year postinjury the distal femur and proximal tibia lost up to 52% and 70% of their BMD respectively[64].
pQCT scans during this phase showed a significant decline in tibial trabecular BMD (at 6 and 12 months) as well as in cortical BMD (only at 12 months)[74,85]. Other epiphyseal (BMC, total BMD) and diaphyseal (BMC, cortical CSA) parameters of the tibia and femur bones also decreased[74]. Lower trabecular BMD was reported in the radius and ulna (at 6 & 12 months) and cortical BMD (at 12 months) in people with tetraplegia with no differences detected at these sites in those with paraplegia[85]. pQCT results in acute SCI further emphasise the rapid rate of trabecular loss compared to cortical loss.
Chronic SCI
Significant decreases in BMD and BMC have been documented at the distal femur[86-88], proximal tibia[86,87,89] proximal femur[14,90], femoral neck[54,87,88,91,92], femoral shaft[87] and total femur[88]. This BMD loss seemingly occurs in the lumbar spine as well[52,93], but was previously undetectable by conventional DXA[14,94]. This was likely due to an overestimate of bone mass resulting from neuropathic calcification and other skeletal abnormalities in the vertebrae of people with SCI[52].
pQCT scans in people with paraplegia revealed significantly lower tibial total BMD[80,82], trabecular BMD, cortical BMD, and cortical thickness, as shown in Figure 2, alongside similar periosteal and increased endosteal circumferences[82] compared to controls. These findings in cortical parameters further confirm the proposed mechanism of cortical thinning by endocortical resorption[55].
BMD and BMC at the distal and proximal tibia epiphyses[77,90] and in the patella have been reported to be significantly less than those in controls. Sabo et al. reported that the cortical area at the distal femur and proximal tibia was lower than that in controls but no difference in cortical BMD was reported in this study[90]. The loss in volumetric BMC was more pronounced at the distal and proximal tibia than in the diaphysis[77,82].
Factors influencing rate and magnitude of bone loss following SCI
It has been shown that there are inter-individual and site-specific differences in the rate of bone loss after SCI (Figure 3), with some individuals approaching published BMD fracture thresholds within only 1 year post-injury (around 67% loss in the distal tibia trabecular BMD) while others experience minor BMD reductions within the same period (around 1% in distal tibia trabecular BMD)[76]. Intra-individual differences have also been reported in another study where bone loss was greater at the proximal tibia compared to the distal tibia and more pronounced in the epiphyses than in the diaphysis (Figure 2)[80], with evidence that inter-site variance in bone loss may be related to bone geometry[74,80].
Figure 3.
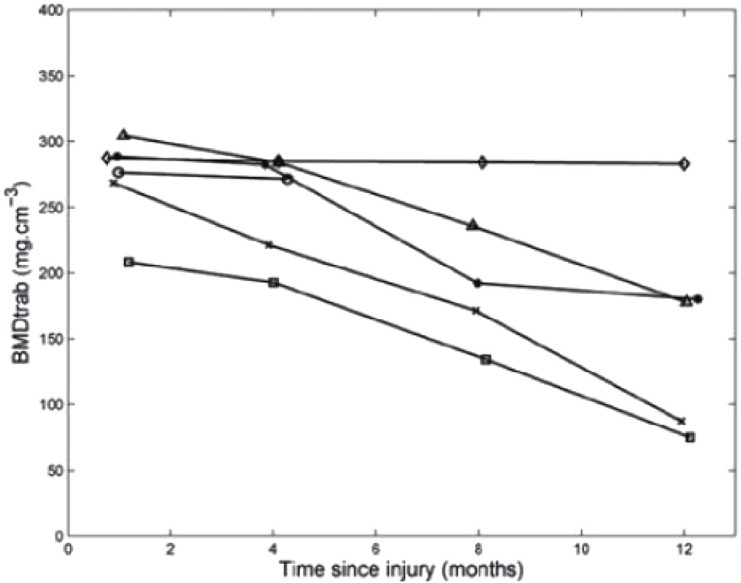
Trabecular BMD of the distal tibia measured in six participants shortly after complete SCI, and 4, 8 and 12 months postinjury[76].
An inverse relationship was found between BMD and time since injury[83,88,95]. Unsurprisingly, bone loss has also been found to be influenced by the type, level and completeness of injury (and resulting function). Lower BMD has been reported in the upper extremities[55,63,95] and lumbar spine[63] in people with tetraplegia, compared to those with paraplegia. Patients with complete SCI have been found to have significantly lower BMD than those with incomplete injuries (BMD= -2.29±0.51 in complete versus -0.12±0.22 in incomplete, P<0.05)[86,95]. Bone loss has also been found to be influenced by the level of injury so that individuals with higher spinal lesions tend to have lower BMD (in the affected skeletal sites) compared to those with lower lesions[56,87,93], but this primarily depends on the injury completeness. While it is likely that function linked to lesion level affects the extent of bone loss, to date these associations have not been reported in the literature.
Neither age[78,86] nor sex have been found to have an effect on bone parameters after SCI[74]. However, a significantly lower cortical BMD has been reported in females compared to males in one study, suggesting a possible effect of gender on cortical bone parameters which was proposed to be influenced by the postmenopausal females participating in this study[74]. The possible effect of spasticity on BMD seems to be unclear with two studies reporting less loss in BMD in spastic compared to flaccid patients[95,96] and other studies reporting no differences in BMD[85,87,97,98] between the two groups. However, in one of the studies that reported a positive effect of spasticity on BMD[95], the 41 tested participants were a mixture of individuals with paraplegia and tetraplegia, and complete and incomplete SCI. It is clear that the 2 groups (spastic and flaccid) were not matched for these influencing factors.
Fragility fractures after SCI
Osteoporosis is characterised by reduced BMD and deterioration in bone micro-structure that consequently leads to reduced bone strength and increased susceptibility to fracture[49]. The link to fracture risk has been demonstrated as bone geometry parameters[73] and BMD were found to be lower in participants with SCI who had lower limb fractures compared to those with no fracture history[6,15,73,87].
Fragility fractures are common and unresolved consequences of SCI[57] and are mostly caused by minor trauma[99] during transfer between surfaces or turns in bed[100-102] or even during rehabilitation training sessions[103,104]. They are more frequent in patients with SCI compared to the general population[14] and occur predominantly in the lower extremities[15,57]; fewer[105] or even no fractures have been reported in the upper limbs[57,62]. The majority of these fractures occur in the femur and tibia[99] especially at epiphyseal sites at the ankle and knee joints[57,105]. This can be linked to the more dramatic and rapid rate of bone loss documented in these trabecular bone-rich site compared to that in the cortical-rich shaft.
In general, people with SCI are more likely to sustain bone fractures in the chronic phases starting at a mean of 3 to 8.9 years postinjury[15,62,99] (Figure 4). Annual fracture rates of individuals with a SCI are double those in uninjured (2% and 1% respectively)[62] but this additional risk is highly site-specific. People with SCI have a 23-fold higher risk of experiencing femur fractures whereas upper limb fracture risk is lower compared to controls[62].
Figure 4.
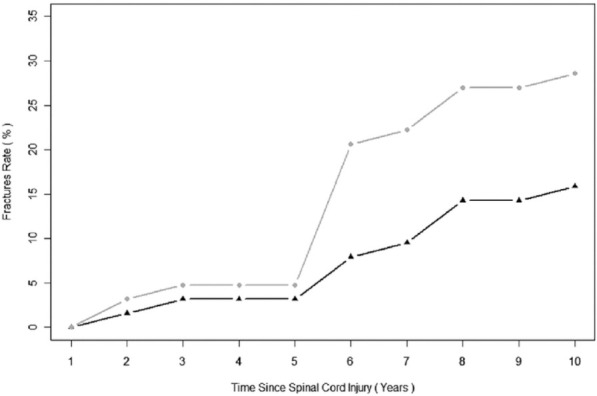
Cumulative rate of fracture recorded during the first 10 years postinjury. The black line with triangles represents the rate of patients sustaining new fractures while the grey line with circles represents the rate of newly sustained fractures per patient[99]. (Reproduced with permission).
Fractures risk factors
Fractures are more frequent in women and also in men with a family or personal history of fractures[62,106]. Fracture risk increases with time since injury[99,106], reaching 4.2% per year in individuals who sustained an SCI more than 20 years ago[15]. Severity of SCI has also been found to be a contributing risk factor, with fractures found to be more common in patients with complete SCI than in those with incomplete injuries[99,105,106]. These factors have been shown previously to contribute to the magnitude of bone loss following SCI, illustrating the correlation between severity of bone loss and fracture risk in these patients. Other factors associated with high risk of fracture include white ethnicity[106] and higher alcohol consumption[105].
It is worth pointing out that our understanding of many aspects of fragility fractures after SCI such as risk factors and fracture thresholds, is limited by the few studies addressing these issues. More studies should be carried out to obtain clarity which should result in the development of effective interventions.
Complications of fractures
About 50% of fractures in individuals with SCI are associated with clinical complications[99,105] such as infections, pressure ulcers[101,105], delayed healing, autonomic dysreflexia, increased muscle spasticity and depression[105,107]. Delayed union can lead to further surgical interventions and therefore prolonged hospitalisation and increased cost[57].
Effective interventions that target bone health and attenuate the rapid decline in bone microstructure and geometry should be incorporated into the patient’s treatment plan as soon as they are clinically stable. However, there is currently no evidence that supports the effectiveness of any intervention in preventing fragility fractures.
Therapeutic Interventions targeting bone loss after SCI
Different physical and pharmacological interventions have been tested in patients to investigate their effectiveness in reversing or attenuating bone loss in the acute and chronic phases, respectively.
Interventions based on electrical stimulation (ES)
Different ES-induced interventions have been employed to improve muscle and bone health and attenuate their deterioration after SCI by eliciting muscle contractions and thereby restoring bone loading. Functional electrical stimulation (FES) techniques applied to the paralysed limbs of people with SCI use surface electrodes to either activate one muscle group to produce joint extension or flexion[108-112], or induce co-ordinated contractions of two or more muscle groups to produce functional movements such as cycling and rowing[113-117]. While pronounced improvements in body composition[118,119] muscle geometry[114,115,120,121] and functional properties[108,111,121-123] have been widely documented, the evidence for the efficacy of FES interventions in attenuating bone loss is equivocal.
Significant site-specific improvements in BMD (ranging between 7-30% increase in BMD) have been reported in some of these studies[109-111,115-117] as shown in Figure 5. In contrast, others found no effect on BMD after undergoing 5-12 months of electrical stimulation-induced training[108,112,113]. Table 1 provides a summary of all studies that used ES- interventions to attenuate the loss in BMD after SCI.
Figure 5.
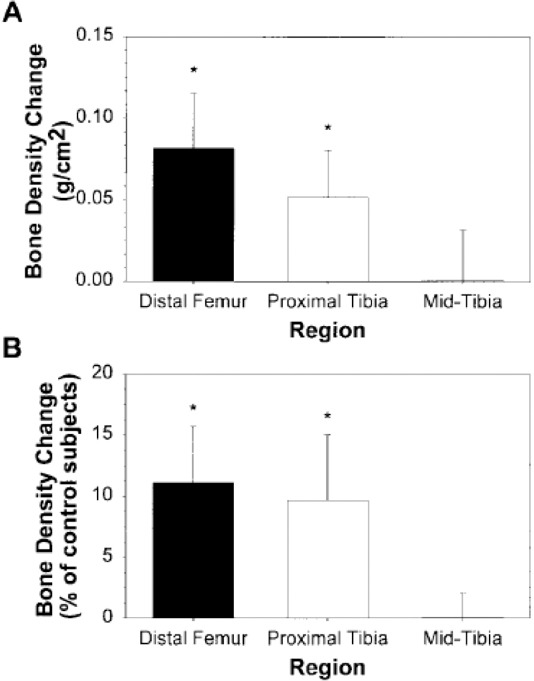
Changes in BMD at distal femur, proximal tibia and mid tibia (as absolute values in A and as percentages of uninjured controls values in B) after undergoing 6 months of ES-knee extension intervention[111]. (Reproduced with permission).
Table 1.
Summary of studies that used ES- interventions to attenuate the loss in BMD after SCI.
| Study | Training Modality | Electrical stimulation parameters | Produced Stress/power | Training dura-tion/frequency | Injury duration and level | Imaging modality | Changes in bone parameters | Level of evidence |
|---|---|---|---|---|---|---|---|---|
| Pacy et al., 1988[130] | Leg raising against load + bicycle ergometer | 6s-6s stimulation-rest, 300μs, 40 Hz. 65-90 V, for leg raising against load ranging from 1.4-11.4 kg, and 80-125 V for bicycle ergometry. | From 0 to 18.75 W (0-3/8 kilopond) | 15 mins, 5 times/week for 10 weeks (leg raising) -15 mins, for 32 weeks (bicycle ergometry), 50 rpm | 1, 3 and 4 years, T4-T6 (3 SCI patients) N.B.: 1 patient had hemangio-blastoma at T6 for 6 years | DXA | No change in BMC or density | - |
| Rodgers et al., 1991[128] | FNS-induced knee extension (KE) | Progressive resistance load on ankle 0-15 Kg | - | 6 KE/min/leg, 3 times/ week for 12-18 weeks, | 6.4±6.1 years, C4-T10 | QCT | No change in BMD | Poor |
| Sloan et al., 1994[123] | FES-cycling (Also participating in physiotherapy) | - | 50-60 rpm for patients with incomplete and 30-40 rpm for complete SCI | 30 min, 3 times/week, for 3 months | 0.2-11.6 years, C5-T12 | DXA | No change in BMD (BMD tested in only 2 out of 12 patients) | Poor |
| Bloomfield 1996[125] | FES-cycle ergometry | Μonophasic, 350 msec duration at 30 Hz and up to 130 mA | Cycling power up to 18 W | 30 mins, 3 sessions/week, (80 sessions) for 9 months | 6 years, C5 to T7 | DXA | Increased by 0.047±0.010 g/cm2 at the lumbar spine; 78% increase in serum osteocalcin, PTH increased 75% but then declined to baseline | Fair |
| Mohr et al., 1997[114] | FES-cycling | - | Workload 1/8 Kp- 7/8 Kp, 18±2 KJ/session | 30 min, 3 days/week, for 12 months followed by 6 months of 1 session/ week | 12.5±2.7 years, C6-Th4 | DXA | 10% increase in PT BMD. This gain faded after 6 months of reduced training | Fair |
| Belanger 2000[111] | Quadriceps contraction (resisted & unresisted) | 300-μsec rectangular pulses delivered at 25Hz with a 5-sec on/5-sec off duty cycle | 40 Nm | 1-hour a day, 5 days a week, for 24 weeks. | 9.6±6.6 years, C5-T6 | DXA | 30% of lost BMD recovered in distal femur and proximal Tibia -Large strength gain | Poor |
| Eser et al., 2003[113] | FES-cycling and passive standing (2 days/week) | Πeak current =140 mA. Pulse width set 0⋅3- 0⋅4 ms, frequency set at 30, 50, and 60 Hz | Power output between 0 and 1 kiloponds | 30-min, three times a week for 6 months | 4.5 weeks | CT | No effect in tibial cortical BMD | Fair |
| Chen et al., 2005[127] | FES-cycling | 20 Hz; pulse duration, 300 μsec; up to 120mA | - | 30 minutes/day, 5 days/week, for 6 months | At least 2 years and 7 months, C5- T8 | DXA | BMD at DF and PT increased 11.13%, and 12.92% respectively, but decreased at FN | Fair |
| Shields et al., 2006[110] | ES-isometric plantar flexion | 10 pulse train (15 Hz, 667ms) every 2 seconds | Compressive loads: 600 N (90% BW) to 1,107 N (150% BW) | 4 bouts/day, each consisting of 120 trains, 5 days/week, for 3 years | 4.5 months, C5 and T12 | DXA | Decline in trained tibial BMD (10%) less than the untrained (25%) | Fair |
| Shields and Dudley-Javoroski 2006[122] | ES-isometric plantar flexion | 0-200 mA, 400 V, 10 pulse train (15 Hz, 667ms) every 2 seconds | ~1-1.5 times BW | 4 bouts/day, each consisting of 125 trains, 5 days/week, for ≥2 years | 6 weeks, ASIA A | pQCT | 31% higher distal tibia Trabecular BMD compared to untrained limb | Fair |
| Clark et al., 2007[112] | ES of quadriceps and dorsiflexors | 30 Hz, (tetanic) stimulation: rest ratio 4:8 s, supine position, knee flexed at 20° | - | 15 min sessions, twice daily, over a 5-day/week, for 5 months | 3 weeks, C4-T10, (all with tetraplegia) | DXA | Different total body BMD at 3 months only | Fair |
| Shields and Dudley-Javoroski 2007[108] | ES -isometric plantar flexion | 0 to 200 mA at 400 V, 10-pulse train (15 Hz; 667ms) every 2s (125 trains in each stimulation bout) | Compressive loads equivalent to 110% of body weight | 30 min/day, 5 days a week, for 6-11 months | >2 years ASIA A | DXA | No change in proximal tibia BMD | Fair |
| Frotzler et al., 2008[115] | FES-cycling | 50 Hz, pulse width = up to 500 μs, current amplitude = 80-150 mA | - | 58±5 min, 3.7±0.6 sessions/week for 12 months | 11.0±7.1 years | pQCT | Increases in distal femoral epiphysis BMD are: 14.4±21.1% in trabuclar BMD, 7.0±10.8% in total BMD and 1.2±1.5% in CSA | Fair |
| Griffin et al., 2009[119] | FES-cycling | 50 HZ, up to 140 mA, 49 rpm | 0.71-10.51 W | 30 min, 2-3 times/week for 10 weeks | 11±3.1 years, C4-T7 | DXA | No difference in bone mass | poor |
| Lai et al., 2010[117] | FES-cycling | 20 Hz; 300 μsec, (electrodesat mid quads and hamstirngs) | - | 30 min, mean of 2.4 sessions/week, for 3 months | 26-52 days, C5-T9 | DXA | Decreased rate in distal femur BMD less in trained group (2.23% in trained; 6.65% in controls) | Fair |
| Dudley-Javoroski et al., 2012[109] | Compressive loads applied during stance by quadriceps ES | 60, 100-pulse trains (20 Hz, 200 μs, up to 200 mA), each train followed by 5 s rest | 150% body weight (BW) | 30 mins, 5 days a week for 3 years | 0.19- 24.23 years, C5-T12 | pQCT | BMD in limbs that received 40% BW and untrained was 61.1% of that of 150% BW limbs | Poor |
| Gibbons et al., 2014[129] | FES-rowing (1 participant) | 50 μsec pulse width, 50 Hz, up to 115 mA unramped stimulation. | - | 30-45 mins, For > 8 years | 13.5 years, T4 | pQCT | PT trabecular BMD was higher in trained participant compared to SCI group but less than able-bodied. | Single case study |
| Gibbons et al., 2016[116] | FES-rowing | Lower limbs exposed to ~2700 loading cycles/week | - | 3 times/week, 30-min rows at 30 strokes/min, | 13.5 years, T4 | HR-pQCT | Majority of tibial trabecular and cortical measurements were within ~1 s.d. but strength was lower. | Single case study |
| Johnston et al., 2016[131] | FES-cycling (compare low and high cadences) | 250 μs, 33 Hz, and up to 140 mA | Low: 20 rpm, 2.9±2.8Nm, High: 50 rpm, 0.8±0.2 Nm | 56 min, 3 times/week for 6 months | 1-27.5 years, C4-T6 | DXA and MRI (microstructure) | Greater decreases in alkaline phosphate and N-telopeptide in low cadence | Fair |
This discrepancy in the documented results of FES on bone health might be due to different factors related to the patient population, intervention protocols and the imaging modalities used to assess changes in bone[124]. For example, a patient’s postinjury duration and level of injury seem to influence intervention effectiveness considerably. Studies showed that starting FES interventions within the first few weeks (1-7 weeks) after SCI was effective in attenuating BMD decline in trabecular-rich regions of the femur and tibia[117,122] but with no effect on cortical BMD[113]. Individuals with paraplegia seem to achieve greater FES-cycling power when compared with people with tetraplegia (22.5 W and 4.8 W, respectively)[125]. This is likely due to the preserved control of their upper body which enables them to better coordinate movement with the pedalling action of the lower limbs and thereby delay fatigue in the leg muscles.
The magnitude of the elicited muscle forces and the session duration and frequency also influence the intervention results. This is probably because they determine the magnitude of the mechanical loads acting on bone which need to be large enough to exceed the remodelling threshold and induce bone formation[126]. BMD has been reported to be significantly greater in patients who trained at higher cycling power (≥18 W)[125] or received larger compressive loads (150% body weight)[109], compared to that measured at the same sites in patients who trained at lower cycling power (≤12 W) and compressive loads (40% BW), respectively. Training paralysed muscles for at least 1 hour per day[111,115], for 5 days per week[109-111,127] seems to be more effective in attenuating BMD loss compared to a training protocol of 30 min, or 3 days per week[113,119,123,128]. Furthermore, significant improvements have been shown from training interventions that have lasted at least 6 months[109-111,114,115,122,125,127,129] compared to those lasting less than 6 months and reported no change in bone mass[119,123,128,130]. This suggests that bone’s response to such interventions is highly dose-dependent.
The magnitude of muscle forces elicited by ES has not been reported in many of these studies[112,115-117,127-129]. However, some studies measured indirect indicators of muscle force such as joint torques (40 Nm[111] and up to 3 Nm[131]) and applied loads as percentages of body weight[108-110,122] (110%, 150%, 90-150% and 100-150% BW, respectively). These achieved loads are comparable to the compressive forces applied on the knees of uninjured individuals during daily activities such as stair descending (123.58% BW) and walking (101.03% BW)[132]. It has also been suggested that a minimum knee extensor torque of 50 Nm is required to achieve standing and walking using FES[111] and at least 35.3-49.2 N/kg muscle force of the knee and hip extensors is required to achieve a sit-to-stand movement[133].
Other studies reported the achieved cycling cadences[123,131] (ranging between 20-60 rpm) and power[119,125,130] (10-18.75 W) as an outcome measure. However, these are not accurate indicators of muscle force and thereby bone loading, because of the inverse relationship between force/load and contraction velocity in skeletal muscles. This concept is demonstrated by considering that high cycling power can be achieved by exerting low force at higher speed as well as by high force and lower speed[109,110]. It is the latter form of high-power cycling (high force, combined with a low velocity) that has been shown to improve bone parameters most effectively in people with SCI[131].
These different ways of measuring the outcome of the aforementioned interventions do not lend themselves well to quantitative comparisons, in terms of the muscle forces produced. Nevertheless, a qualitative comparison between different training approaches (cycling, rowing and resistance training) can be made. Whereas cycling requires stimulating the quadriceps, hamstrings, gluteal muscles and (in some studies) calf muscles, most of the resistance training studies targeted one muscle group such as the quadriceps[109,111] or the soleus[110]. Stimulating one muscle group has been found to mitigate the loss in BMD asymmetrically, on one side/half (posterior) of the lower limb long bones[134,135]. Accordingly, it might be speculated that stimulating the antagonist muscle pairs could have a more homogenous effect on BMD throughout the different areas of the bone.
The imaging modalities used to evaluate the effectiveness of FES interventions are another important methodological consideration. DXA does not distinguish between trabecular and cortical bone and thereby is not able to detect early potential changes in response to training, which are known to occur more rapidly in trabecular than in the cortical compartments[115]. This is illustrated in Figure 6, which shows the effect of training on femur trabecular bone using CT[20], with no apparent differences in the cortical shell. Moreover, DXA does not typically provide site-specific scans for regions such as distal femur and proximal tibia which are of clinical interest and are likely to be stressed by cycling exercise.
Figure 6.
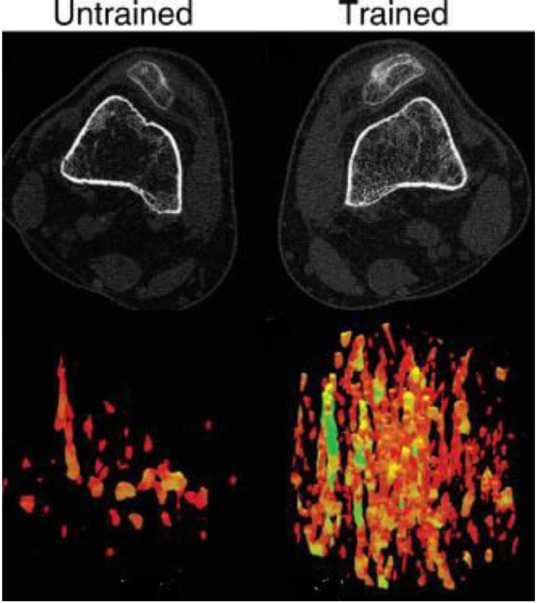
Upper panel: CT images of an untrained (left) and trained (right) limbs at 12% of the femur length. Lower panel: 3D reconstruction of the trabecular lattice at the same region showing the greater loss in the untrained compared to the trained limb[109]. (Reproduced with permission).
Only one study estimated the effect of ES-training on bone strength[116], reporting increases in multiple trabecular and cortical parameters after ES-rowing intervention. However, bone strength (which was estimated using computational modelling from biomechanical indices such as stiffness (-3 SD) and predicted failure load (-3.5 SD)) was lower than non-SCI controls; this was proposed to be related to the larger percentage cortical porosity (+4.6 SD) and mean pore diameter (+3.7 SD)[116]. More studies are required to investigate the effect of ES-interventions on bone strength alongside structural and geometric parameters (as no effect was found in the latter in one study[114]) to assess their effectiveness in preventing fragility fractures.
Different forms of ES-interventions seem to partially reverse BMD when applied intensively over the long-term in people with chronic SCI. However, these improvements have not been shown to lead to fracture risk reduction (which would be considered the desired and clinically relevant outcome). The biomechanical loads/forces elicited by these interventions should be measured and further tested to find out how could they be delivered effectively and safely. Ideally, the ES-interventions would mimic the voluntary muscle loading exerted by able-bodied individuals during daily activities. Additional research is also needed to determine whether it is possible to identify a ‘loading dose threshold’ above which fracture risk could be significantly minimised.
Other physical interventions
Weight bearing
Other forms of physical training interventions include weight-bearing activities such as standing and walking, which aim to load the lower limb bones through axial compression, bending and torsional stresses that would normally act on the lower extremities during standing and ambulation.
Most of the reviewed studies (11 out of 16 studies) based on conventional standing training (using standing frames, wheelchairs and leg braces)[64,87,136-139], exoskeletons[140-143] or treadmill walking[144,145] did not show improvements in BMD, either during the acute phase[137,139,144] or during the chronic phase[64,87,136,138,140,141,145]. Only four studies showed positive results in bone parameters by preventing bone loss in the acute to sub-acute phase (standing and treadmill walking)[146] and increasing BMD in the chronic phase (treadmill training)[147] (passive standing)[148,149]. The participants recruited were full-time wheelchair users with complete SCI in some of these studies[136,139-142,148,149], or had incomplete injuries (or a mixture of both) in other studies[64,87,137,138,144-147]. Only three studies reported that the recruited participants were already physically active before their recruitment[144,147,149]. Two of these studies reported a positive impact of the intervention on BMD[147,149], which suggests that being active might have improved their muscle health and attenuated their atrophy which in turn optimised their force production and subsequently their impact on bone stimulation.
These improvements range from 7-9% larger BMD at different sites in the lower limbs in standing groups compared to controls. However, one of these studies was a single case study recruiting a subject with motor-incomplete SCI (achieving a 20% increase in tibial trabecular BMD)[147], which in itself is known to lead to less bone loss than complete SCI[86,95]. Goemaere et al reported an improvement in femoral shaft BMD but not in the proximal femur[149]. These results were explained by a possible difference between cortical and trabecular bone in the minimal effective strain for initiating bone remodelling (being reached more rapidly in cortical bone)[149], although recent evidence suggests that there are regional variations (regardless of bone type) in strain thresholds within the same bone[150]. In this study, however, the effect of standing was not investigated at the sites that are known to be most prone to fragility fractures in people with SCI (distal femur, proximal tibia and distal tibia). The last study reported attenuation of bone loss based on bone formation and resorption biomarkers but BMD results did not always match the biomarker results[151].
To summarise, to achieve significant improvements in BMD in the lower limbs from standing and walking, long-term training sessions should start within the first few weeks after the injury onset[146,148]. Combining ES with weight bearing activities may have a greater positive effect on bone than performing these activities on their own[147]. While the duration of the ES-training has been shown to have an impact on BMD[152], standing for different durations (<1 hour, 1 hour and >1 hour)[64,138] and frequencies (daily standing versus standing for 3 times/week)[149] seems to have no significant effect on BMD. This might indicate that the compression stresses produced during standing alone are insufficient to stimulate lower limb bones even when applied for longer durations.
Similarly, bone stresses delivered through treadmill walking seem to be insufficient to induce adequate bone stimulation. This is, likely because of partial bodyweight support and low treadmill speeds used compared to normal walking speed[144], which would result in lower bone strains[153]. It should also be noted that the atrophied muscles of patients with SCI would produce smaller bone strains when contracting which would make them less effective in inducing osteogenic effects in the bones of the paralysed limbs[142]. Again, as seen with FES interventions, the dosage of the mechanical loading acting on bone that is required to attenuate or reverse bone loss effectively is yet to be ascertained[154].
Additional studies are also needed to determine whether partial body-weight supported treadmill training and other gait rehabilitation orthoses are more effective in terms of bone stimulation than conventional training[144] and whether they could be used safely for long term intervention, as home-based rehabilitation devices[141].
Ultrasound
As high-frequency mechanical waves, ultrasound was thought to be a potential technique to stimulate bones with mechanical signals. This concept is based on results of a number of in-vitro trials where low-intensity pulsed ultrasound has been reported to induce osteogenic responses[155,156]. However, in the published literature there is only one study that investigated its effect on bone health in people with SCI. The study found no significant effect of applying pulsed ultrasound for 6 weeks on calcaneal bone loss[157], although the short trial duration likely limited the relevance of the study. Further investigation should be carried out to study the effects of varying ultrasound parameters, as well as, the intervention duration and frequency on its effectiveness in treating bone loss after SCI.
Whole Body Vibration
Whole body Vibration systems have been used in people with SCI to deliver vertical or side-alternating oscillations throughout the long bones using a vibrating plate upon which the feet are situated, either while the participant is sitting[158] or passively standing[159]. In the former, the limb is fixed using external compressive loads (35% BW) in order to optimise the transmission and effectiveness of the vibrations[158]. It has been reported that neither 6 months[159] nor 12 months[158] of applying whole body vibration combined with weight-bearing activities has induced any improvements in BMD or microstructure of the lower extremities. Only one study reported an improvement from whole body vibration (when combined with standing) in BMD (using DXA) in the spine (8.3%) and trunk (5.5%), but not in the lower extremities[160].
Whole body vibration has been incorporated into the rehabilitation programmes of some patients with SCI very recently, but the number of studies that have investigated its effect on bone health is very limited. A summary of the studies that investigated the effect of weight bearing exercises, ultrasound and whole-body vibration on BMD after SCI, can be found in Table 2.
Table 2.
Summary of studies that used other physical interventions (without electrical stimulation) to attenuate the loss in BMD after SCI.
| Study | Training modality | Produced Stress/power | Training duration/frequency | Injury duration and level | Imaging modality | Changes in bone parameters | Level of evidence |
|---|---|---|---|---|---|---|---|
| Biering-Sørensen et al., 1988[87] | Standing or walking using long leg braces | - | For at least 1 hour daily | 2-25 years, C7-L3 | DXA | No effect on BMC | - |
| Kunkel et al., 1993[136] | Standing in frame | - | 45 min/twice daily for 5 months (144 h over 135 days) | 10-39 years, C6-T12, (4 SCI, 2 multiple sclerosis patients) | DXA | No change in BMD | Fair |
| Goemaere et al., 1994[149] | Passive Standing using: 1. long leg braces, 2. standing frames, 3. standing wheelchairs) |
- | Daily standing for 1 hour in 1 group and 3 times/week in the second group | 12-118 months. Complete paraplegia | DXA | BMD better at femoral shaft but not proximal femur compared to non-standing | Fair |
| Thoumie et al., 1995[141] | Gait rehabilitation with hybrid orthosis | - | 2 hours, 3 times/week, for 16 months | 15-60 months, T2-T10 | DXA | Significant decrease in BMD at femoral neck and no change at lumbar spine | Poor |
| Needham-Shropshire et al., 1997[142,143] | Standing and walking using a device that combined ES and a modified walker | - | 3 times/week, 12-20 weeks, (mean of 143.6±86.4 mins persession) | At least 6 months, T4-T11 | DXA | No significant change in BMD at FN, neck, and Ward’s triangle | Fair |
| de Bruin et al., 1999[146] | Standing and treadmill walking | Treadmill speed=1.3 km/h | 30min standing, 30 min walking, 5 days/week for 6 months | 1-4 weeks, C4-L1 | pQCT | Almost no loss in tibia trabecular BMD in trained group compared to -6.9% to -9.4% loss in trabecular bone | Fair |
| Dauty et al., 2000[64] | Passive standing | - | Daily for: 1. less than 1 h, 2. 1 h, 3. More than 1h | 68.3±74.7 months | DXA | No effect on BMC | - |
| Frey-Rindova et al., 2000[137] | Standing using frame (for complete SCI) and treadmill walking (for incomplete SCI) | - | At least 30min, 3 times/week for 2 and half years (treadmill speed 1.3 km/h) | 1-4 weeks | pQCT | No effect on BMD | - |
| Warden et al., 2001[157] | Pulsed US | - | 20 min, 5 days/week for 6 weeks US settings: 10 μsec 1.0 MHz sine waves, 3.3 kHz | 1-6 months, C5-T10 | DXA | No effect on calcaneal bone parameters | Fair |
| Ben et al., 2005[139] | Standing on 1 leg (on tilt table) | 17 Nm dorsiflextion torque | 30 min, 3 times/week, for 12 weeks | 4±2 months | DXA | Little or no effect on femur BMD | Good |
| Giangregorio et al., 2005[144] | Body weight supported treadmill | - | Less than 1hour, 2 times/week (48 sessions in 8 months) (speed= 0.7-2 km/h) | 2-6 months, C3-C8 | DXA and CT | No effect on BMD (proximal and distal femur, PT, spine) or CSA (mid-femur, PT) | Poor |
| Carvalho et al., 2006[151] | Treadmill gait training | 30-50% BW supported | 20 min, 2 times/week for 6 months | 25-180 months, C4-C8 | DXA | Most of the participants showed increased bone formation and decreased bone resorption (BMD results did not always match biomarkers results) | Poor |
| Giangregorio et al., 2006[145] | Body weight supported treadmill | - | 3 times/week, For 12 months (144 sessions) | 1-24 years (all Incomplete) | DXA and CT | No effect on BMD (at proximal and distal femur, PT, spine) or CSA (mid-femur, PT) | Poor |
| Alekna et al., 2008[148] | Passive standing in frame | - | For at least 1 hour/day, no less than 5 days/week | 8-12 weeks, C2-L1 | DXA | Higher BMD in lower limbs after 2 years in standing group (1.018 compared to 0.91 g/cm2) | Fair |
| Goktepe et al., 2008[138] | Any form of Standing: 1. More than 1hour, 2. Less than 1hour, 3. No standing | - | Daily standing | At least 1 year, ASIA A, B | DXA | No significant difference between groups in BMD at PT and lumbar spine | Fair |
| Coupaud et al., 2009[147] | Partial body-weight supported treadmill training (BWSTT) + FES on one side (bisphosphonate + Vitamin D prescribed independently) | 30% BW support -Speed increased from 0.1 m/s to 0.3 m/s | Muscle conditioning over 2 months Target increased for 15 min to 30 min, 3 time/week for 5 months. FES: 40Hz, 40mA, and 117-351μs | 14.5 years, T6 (incomplete) (one subject) | pQCT | Increase of 5% (right) and 20% (left) in DT trabecular BMD. Changes are negligible in PT and DF | Single case study |
| Davis et al., 2010[160] | 3 phases of training: 1. standing only, 2. partial standing/WBV (foot only on plate), 3. standing with vibration |
- | 1. Phase 1: 40 min, 3 times/week, for 10 weeks, 2. Phase2: 20/20 mins, 3 times/week, 3. Phase3: 7 mins/session, 3 times/week |
4 years, T10 (incomplete), (single case) | DXA | Improvement in BMD in the trunk and spine after phase 3 only. No effect on legs |
Single case study |
| Wuermser et al., 2015[159] | Low-magnitude whole body Vibration+ passive standing | About 76-86% BW | 20 mins, 5 days/week, for 6 months (0.3 g, 34 Hz,50 μm.) | 2-27 years, T3-T12 | DXA and HR-pQCT |
No effect on BMD at PF or microstructure at DT | Fair |
| Dudley-Javoroski et al., 2016[158] | Body Vibration | 35% BW applied during the vibration training | 3 times/week, for 12 months Vibration parameters: 0.6g, 30 Hz, 20 min, three times weekly) | 0.1 to 29.2 years, C7-T4 | pQCT | No effect on trabecular microstructure or BMD at DT and DF | Fair |
| Karelis et al., 2017[140] | Walking with a robotic exoskeleton | - | Up to 60 min, 3 times/week for 6 weeks Mean standing time/session: 48.4 min, walking time: 27.0 min | 7.6 ± 4.6 years, C7-T10 | DXA | No significant change in BMD | Fair |
To summarise, most of the physical interventions had limited effects on bone health. This may be related to an inability of current methods to develop or safely apply large internal muscle forces or external forces to bone. For those with chronic SCI, treatments aimed at reversing osteoporosis should be considered, but are less likely to be effective in restoring BMD values to within the normal range[152]. This has been proposed to be due to the weaker bone losing its ability to adapt to applied strains[108] which might be due to the cellular accommodation phenomenon[161] or osteocyte apoptosis[162]. Moreover, patellar tendon stiffness was found to be reduced by up to 77% in people with chronic SCI compared to uninjured controls[163]. This would make force transmission from muscles to bones through tendons more difficult and less efficient[163].
Pharmacological interventions (Bisphosphonates)
A wide range of pharmacological treatments for osteoporosis are currently available, such as strontium ranelate[164], denosumab[165], selective oestrogen receptor modulator drugs[166] and bisphosphonates. The latter are the most commonly prescribed treatments for osteoporosis in women[167-169] and men[170,171], and have been found to be effective in attenuating bone resorption, restoring BMD and preventing fractures[172].
Bisphosphonates are anti-resorptive agents that reduce the bone resorption rate by targeting osteoclasts, inhibiting their activity and subsequently reducing their number in the long term[173]. They are administered either orally on a daily or weekly basis[174] or as an annual single[67,175] or multiple (every month or 3 months)[176,177] intravenous injection. Patients taking bisphosphonates on a weekly basis have been found to be more compliant and to persist with the treatment compared to those who take a daily dose[174].
However, bisphosphonates studies conducted in patients with acute SCI showed mixed results, with 5 out of 9 studies reporting positive effects. Alendronate (weekly for 12 months) has been found to preserve total body and leg BMD[178]. Intravenous bisphosphonates such as zoledronate and pamidronate have been shown to have positive effects on BMD in the lower limbs[177], hip and spine[67,179]. One study reported little effect of disodium dichloromethylene diphosphonate on BMD at the distal tibia[180].
These agents resulted in a lower limb BMD that was 7-17% higher compared to untreated controls. However, it should be pointed out here that in three of these studies, about one third of the participants were classified as having an incomplete SCI (with good preservation of motor function) and were not full time wheelchair users[177-179]. With only two studies reporting positive results in participants with complete SCI[67,180]: one reported only a marginal effect on BMC at the distal tibia (7%)[180] while the other reported about 12% greater BMD at the hip[67]. The reduction in bone loss in the acute phase after pamidronate and zoledronate administration (administered once a year) has been reported in some studies to be temporary, lasting 6 months (after the annually administered dose)[175] although the treatment was not discontinued in one study[176]. This might indicate that the dose or the frequency of administration could be further investigated to prolong their effect. Another study reported no effect of the treatment at the knee which might suggest that such treatment is effective in attenuating cortical but not trabecular bone loss[173].
Based on the results reported in these studies, there is little current evidence that supports the effectiveness of bisphosphonates in attenuating/preventing bone loss in the acute phase of complete SCI at the lower extremities. It was apparent that most studies did not investigate the effect of these treatments in the sites that are most prone to fracture, in part because these sites were only identified relatively recently. However, a number of these studies have been carried out since the fracture-prone sites after SCI were identified and published[15,55,181] and so, this is unlikely to explain all the lack of relevant evidence for the effectiveness of pharmacological intervention to treat bone loss after SCI. Furthermore, these findings are possibly due (in part) to the measurement technique used and the lack of studies that investigated these sites using a 3D imaging technique (e.g. pQCT). Positive changes might have been achieved in the trabecular bone (which responds to interventions faster than cortical bone), but it was not possible to observe it with DXA imaging.
Fewer studies investigated the use of bisphosphonates in the chronic phase of SCI[173,182,183] and one study included a mixed patient group with acute and chronic SCI[184]. The effects of alendronate on leg BMD seem to be influenced by treatment duration. A daily dosage of alendronate administered orally had no effect on lower limb BMD[171] over 6 months, but it attenuated bone loss by 9%[184] when administered for 2 years.
As one of the only two studies that reported significant effects of bisphosphonates in the chronic phase, was a single case study of a patient with incomplete SCI[182], it can be concluded that there is no sufficient data to assess their effectiveness for osteoporosis in chronic SCI. It is clear that the positive effect of bisphosphonates on BMD in the chronic phase of SCI is limited when used as the only intervention. Furthermore, and crucially, it does not exceed keeping BMD within its current level without restoring what has been lost, which could be less effective in the chronic phase (after reaching steady state) compared to the acute phase
A number of issues can be highlighted after reviewing the use of bisphosphonates in people with SCI. Firstly, most of these studies tested these treatments in patients with different ambulatory capabilities and injury severity[173,177-179,185-187]. Some of these studies showed more pronounced improvements in ambulatory compared to non-ambulatory participants[177,187]. Despite the fact that there appear to be comparable numbers of studies with mixed and complete SCI that reported positive results of pharmacological interventions (4 studies with mixed group (complete and incomplete SCI) and 3 studies of complete SCI only), testing these agents on only non-ambulatory patients with complete SCI would give a clearer indication of their effectiveness in restoring BMD and preventing fractures[67,188]. As a minimum, where mixed patient groups are used, completeness of injury should be included in the data analysis and reporting of findings.
Secondly, very few studies investigated the effect of these treatments on BMD at the sites that are most susceptible to fracture in people with SCI[67,180,184], such as the distal femur and proximal and distal tibia[57]. Furthermore, the use of pQCT (instead of DXA) could provide more quantitative insights into whether cortical bone and trabecular bone respond to such agents to similar or different extents.
Although these studies reported no[173,178] or only acute mild side effects[179,186], osteonecrosis of the jaws[189] and spontaneous fractures[190] are considered as rare side effects of using oral bisphosphonates in osteoporotic patients. Other clinical treatments have therefore been suggested to be tested on people with SCI such as the anti-resorptive denosumab and anabolic therapies such as sclerostin antibodies[67]. Gifre et al reported an increase in lumbar and femoral BMD (8% and 3% BMD respectively) after administering Denosumab in people with acute SCI for 12 months[191]. This was the only study testing Denosumab in people with SCI. Table 3 provides a summary of all studies that used pharmacological treatments to attenuate the loss in BMD after SCI.
Table 3.
Summary of studies that used pharmacological treatments to attenuate the loss in BMD after SCI.
| Study | Treatment | Injury duration and level | Dose, Duration & frequency | Imaging device | Changes in BMD | Supplements | Level of evidence |
|---|---|---|---|---|---|---|---|
| Minaire et al., 1981[180] | disodium dichloromethylene diphosphonate | Acute SCI, T1-T12, (all with complete paraplegia) | 400 or 1600 mg/day for 3.5 months | Photon absorptiometry | Little effect in BMC at distal tibia (for 400 mg) | - | Fair |
| Pearson et al., 1997[187] | Cyclical Etidronate | Within 6 weeks, C5-T12 | Orally 800mg/day for 2 weeks, this was repeated after 13 weeks | DXA | BMD maintained only in ambulatory treated patients | - | Poor |
| Nance et al., 1999[177] | Intravenous Pamidronate | 6 weeks, C4-T12 | 30-mg infusion/month for 6 months | DXA | Greater BMD at hip, femoral and tibial diaphyses, femoral and tibial epiphyses (less bone loss in ambulatory) | Calcium: 1000 mg daily | Poor |
| Sniger and Garshick, 2002[182] | Alendronate | 27 years, C4 (incomplete), (single case) | Daily: 1. Alendronate: 10mg, 2. Vitamin D: 400mg, 3. Calcium carbonate 500mg, daily for 2 years | DXA | Increased BMD at spine and lower legs | Vitamin D: 400 mg/d Calcium carbonate: 500 mg/d | Single case study |
| Zehnder et al., 2004[184] | Alendronate | 0.1-29.5 years, T1-L3, (all with complete SCI) |
10mg + 500 mg calcium daily for 24 months | DXA | BMD at distal tibia, tibial diaphysis and total hip remained stable compared to control group | Elemental calcium: 500g/d | Fair |
| Bauman et al., 2005[176] | Intravenous Pamidronate | 22 to 65 days, ASIA A, Acute SCI |
60 mg given at 1, 2, 3, 6, 9, 12 months | DXA | No changes in long term (12, 18,24 months) although reported early (1,3,6 months) reduction in bone loss in total leg BMD | Calcium: at least 700 mg/d in diet | Fair |
| de Brito et al., 2005[173] | Alendronate | 13.1-255.7 months, ASIA A, B, C | 10 mg (+1000 mg Calcium), daily for 6 months | DXA | General increase in BMD | Calcium: 1000 mg/d | Fair |
| Mechanick et al., 2006[186] | Intravenous Pamidronate | Acute SCI, AIS A, B, C |
90 mg over 4 hours (single dose) | - | Reduced bone resorption biomarkers, BMD not tested | -Calcium: 1,000 mg daily, -Calcitriol: 0.25 μg daily | - |
| Gilchrist et al., 2007[178] | Alendronate | Within 10 days, C4-L2 | 70 mg once weekly, for 12 months | DXA | Total and hip BMD was 5.3% and 17% greater in intervention group respectively. Effects sustained for more 6 months after treatment discontinued | - | Fair |
| Shapiro et al., 2007[175] | Intravenous Zoledronate | 10-12 weeks, C2 to T12 | 4 or 5 mg (administered once) | DXA | BMD and CSA increased at proximal femur only at 6 months, and for 12 months at the femoral shaft | Calcium: 800 mg, Vitamin D: 800 IU (both from diet) |
Fair |
| Bubbear et al., 2011[179] | Intravenous Zoledronate | Within 3 months, C4-L3 | 4 mg (administered once) | DXA | Higher BMD at total hip (12.4%) trochanter (13.4%), and lumbar spine (2.7%) up to 12 months | - | Fair |
| Bauman et al., 2015[67] | Intravenous Zoledronate | Within 3 months ASI A, B (all with compete SCI) | 5 mg (administered once) | DXA | Reduction of BMD loss at the hip but not at the knee | Calcium carbonate: 1250 mg/d Vitamin D: only for participants with levels <20 ng/ml | Poor |
| Haider et al., 2019[185] | Teriparatide (in previous study) followed by oral alendronate | 15±9 years, ASI A, B, C (C1-L5) | Teriparatide: 12-24 months Alendronate: 70 mg once weekly for 12 months | DXA | Significant increase in aBMD at the spine 2.5% and in BMC at femoral epiphysis, metaphysis, and diaphysis, 15%, 7.7%, 3.0%, respectively. - no clear results at the tibia | Vitamin D (cholecalcifer-ol 1000 IU) daily -calcium carbonate: 1000 mg daily | Fair |
| Gifre et al., 2016[191] | Denosumab | 15±4 months, C4-T8 (ASIA 12A, 1B, 1C) | 60 mg every 6 months for up to 12 months | DXA | Increases in lumbar (8%) and femoral BMD (3%) | Calcium and Vitamin D | Fair |
Combination treatment interventions
Despite the reported mitigated bone resorption in the acute phase[67,177,178] and the stabilised BMD in the chronic phase[183,184] in many of these studies , no increase in BMD has been reported, indicating a need for an intervention with an anabolic effect that can be combined (or used sequentially) with anti-resorptive agents to boost their effect[183,192]. The additive effect of anti-resorptive medications combined with the anabolic stimulus of physical activity may explain results from a number of bisphosphonate studies reporting more pronounced improvements in BMD in ambulatory individuals than in full time wheelchair users[177,187]. Furthermore, combining exercises with anti-resorptive therapies has been reported to have a greater effect on BMD compared to using antiresorptives alone in different models of osteoporosis[193,194] but the number of studies examining such approaches is small[195].
Remembering the significant role of mechanical stresses in preserving/losing BMD might suggest that bisphosphonates should be accompanied by physical training in order to achieve optimum benefits in people with SCI[196]. Hypothetically, by combining these interventions, the anti-resorptive agent would target the inhibition of osteoclastic activity, while the skeletal loading could have a role in simulating osteoblasts, thus mitigating the imbalance between bone formation and resorption caused by the SCI. In a recent study, significant increases in geometric cortical bone parameters (cortical bone volume, cortical thickness index, and buckling ratio) were reported at the distal femur and proximal tibia in the group that had FES-rowing training combined with zoledronate administration compared to the group that performed FES-rowing alone[197]. This is clinically-relevant as reductions in these geometric cortical bone parameters after SCI are thought to be associated with osteoporotic fractures in this patient group[197].
The anabolic parathyroid hormone teriparatide has been also investigated in combination with gait training[192], with vibration[198] and following 12 months of bisphosphonates treatment[185]. The first study reported no effect on BMD (i.e. with gait training)[192]. The second study reported that the combination of vibration with teriparatide did not augment its effect on BMD[198], while in the latter(involving combination with bisphosphonates), an increase in BMC ranging between 3-15% was reported in different parts of the femur[185]. Studies that tested these combined treatments in people with SCI are summarised in Table 4.
Table 4.
Summary of studies that used physical interventions combined with pharmacological treatments to attenuate the loss in BMD after SCI.
| Study | Intervention | Protocol | Physical Training parameters | Injury duration and level | Imaging device | Changes in BMD | Level of evidence |
|---|---|---|---|---|---|---|---|
| Gordon et al., 2013[192] | Parathyroid hormone and gait training | 20 μg/day and robotic-assisted stepping for 6 months, followed by 6 months of teriparatide alone | 40 minutes/ session, 3 times/week, at speed 2.0-2.5 km/h, <50% BW support | >1 year, C1-T10 | DXA (hip and spine) and MRI (microarchitecture of distal tibia) | No change in spine and total hip BMD. Positive anabolic effect significant at 3 but not6 months | Fair |
| Edwards et al., 2018[198] | 3 groups: 1. Teriparatide + sham vibration, 2. placebo + vibration, 3. Teriparatide + vibration |
Teriparatide: 20 μg/d Vibration: 10 min/d -Additional 12 months of Teriparatide treatment | Vibration: 30 Hz, acceleration amplitude = 0.5 g | 19±13.8 years, ASIA A, B, C, D | DXA and CT | Increase in groups that used teriparatide: 4.8% - 5.5% increase in spine BMD -Vibration did not augment teriparatide effect. -Small increase in knee cortical bone in all groups. - the additional 12 months of Teriparatide resulted in 7.1-14.4% increase from baseline) | Good |
| Morse et al., 2019[197] | Intravenous Zoledronate and FES-Row | 12-month FES-rowing program and single dose of zoledronate | 30 min, 3 days/week at an intensity of 75% to 85% of peak heart rate | 0.4-37.9 years, 75% had a motor-complete injury | DXA for BMD, QCT | Greater cortical bone volume, cortical thickness index and buckling ratio at proximal tibia and distal femur metaphysis | Fair |
Levels of evidence
After reviewing all available interventions aiming to mitigate bone loss in people with SCI, the quality of these studies was assessed. Only studies that investigated BMD as their primary outcome were included in this assessment, due to the well-documented link between BMD and fracture incidence in people with SCI[6,15]. Furthermore, it is the most commonly measured bone parameter among all studies in the SCI population.
The levels of evidence were assessed following the approach described by Bryson et al., 2009[188] which was based on the Delphi list 9 items. Each of these items (listed in Tables 5,6,7 (provided in Online Resource 1)) was given a subscore of either 1 (not reported), 2 (fair) or 3 (good), except for the randomisation item which was given a score of 0 (if the study was not randomised). Items 5,6,7 were all merged together under one category due to the small numbers of studies that reported blinding of the patient (which was difficult to achieve especially for physical interventions), assessor or the caregiver. All subscores were summed to determine the overall quality rating (poor, fair, good).
All the reviewed studies were either of a poor or fair quality except one study that tested the effect of standing and was of a good quality[139]. For both the ES and the pharmacological interventions studies, 30% were of poor quality while about 70% were of fair quality. Other physical interventions (such as weight bearing) studies showed comparable levels of evidence with 25% poor, 67% fair and 8% good quality. The quality of most of physical interventions studies were found to be limited by the absence of randomisation and blinding which were difficult to achieve due to ethical and practical reasons, respectively. Also, many studies were not controlled, comparing baseline with post-intervention measures, making item 2 (allocation concealment) and item 9 (intention-to-treat analysis) not applicable.
Conclusions
To summarise, this review article has discussed the aetiology, development and consequences of bone loss in people with SCI. Furthermore, the most commonly used imaging modalities to assess bone loss after SCI and available therapeutic approaches have been evaluated.
It is clear that bone loss that develops in the paralysed limbs after SCI is highly site-specific, progressing with different patterns and timelines in cortical and trabecular bone compartments. In addition, rates of bone loss differ substantially between individuals but to date there is little understanding of the mechanisms responsible for this variation. Most of the physical and pharmacological interventions developed and evaluated to date appear to have a limited effect on bone health, but the poor quality of published studies in this area limits our ability to draw clear conclusions. More high-quality observational and interventional studies, with appropriate outcome measures targeting fracture-prone site, are needed.
Acknowledgements
This review was funded by INSPIRE charity foundation and University of Strathclyde.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Furlan JC, Sakakibara BM, Miller WC, Krassioukov A V. Global Incidence and Prevalence of Traumatic Spinal Cord Injury. Can J Neurol Sci. 2013;40:456–64. doi: 10.1017/s0317167100014530. [DOI] [PubMed] [Google Scholar]
- 2.Wyndaele M, Wyndaele J. Incidence, prevalence and epidemiology of spinal cord injury:what learns a worldwide literature survey ? Spinal Cord. 2006;44:523–9. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- 3.Mccaughey EJ, Purcell M, Mclean AN, Fraser MH, Bewick A, Borotkanics RJ, et al. Changing demographics of spinal cord injury over a 20-year period :a longitudinal population-based study in Scotland. Spinal Cord. 2016;54:270–6. doi: 10.1038/sc.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dionyssiotis Y, Mavrogenis A, Trovas G, Skarantavos G, Papathanasiou J, Papagelopoulos P. Bone and soft tissue changes in patients with spinal cord injury and multiple sclerosis. Folia Med (Plovdiv) 2014;56(4):237–44. doi: 10.1515/folmed-2015-0002. [DOI] [PubMed] [Google Scholar]
- 5.Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile - Part I. J Spinal Cord Med. 2014;37(6):693–702. doi: 10.1179/2045772314Y.0000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazo MG, Shirazi P, Sam M, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39(2001):208–14. doi: 10.1038/sj.sc.3101139. [DOI] [PubMed] [Google Scholar]
- 7.Jiang S, Jiang L, Dai L. Mechanisms of osteoporosis in spinal cord injury. Clin Endocrinol (Oxf) 2006;65:555–65. doi: 10.1111/j.1365-2265.2006.02683.x. [DOI] [PubMed] [Google Scholar]
- 8.Giangregorio L, Blimkie CJ. Skeletal Adaptations to Alterations in Weight-Bearing Activity. Sport Med. 2002;32(7):459–76. doi: 10.2165/00007256-200232070-00005. [DOI] [PubMed] [Google Scholar]
- 9.Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, et al. Muscle atrophy and bone loss after 90 days 'bed rest and the effects of flywheel resistive exercise and pamidronate:Results from the LTBR study. Bone. 2005;36:1019–29. doi: 10.1016/j.bone.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Uebelhartl D, Demiaux-Domenech B, Roth M, Chantraine A. Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilisation. A review. Paraplegia. 1995;33:669–73. doi: 10.1038/sc.1995.140. [DOI] [PubMed] [Google Scholar]
- 11.Roberts D, Lee W, Cuneo RC, J. W, Ward G, Flatman R, et al. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab. 1998;83(2):415–22. doi: 10.1210/jcem.83.2.4581. [DOI] [PubMed] [Google Scholar]
- 12.Pietschmann P, Pils P, Woloszczuk W, Maerk R, Lessan D, Stipicic J. I ncreased serum osteocalcin levels in patients with paraplegia. Paraplegia. 1992;30:204–9. doi: 10.1038/sc.1992.56. [DOI] [PubMed] [Google Scholar]
- 13.Maimoun L, Couret I, Mariano-Goulart D, Dupuy AM, Micallef JP, Peruchon E, et al. Changes in Osteoprotegerin/RANKL System , Bone Mineral Density , and Bone Biochemicals Markers in Patients with Recent Spinal Cord Injury. Calcif Tissue Int. 2005;76:404–11. doi: 10.1007/s00223-004-0048-6. [DOI] [PubMed] [Google Scholar]
- 14.Reiter AL, Volk A, Vollmar J, Fromm B, Gerner HJ. Changes of basic bone turnover parameters in short-term and long-term patients with spinal cord injury. Eur Spine J. 2007;16(6):771–6. doi: 10.1007/s00586-006-0163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zehnder Y, Thi ML, Michel D, Knecht H, Perrelet R, Neto I, et al. Long-term changes in bone metabolism , bone mineral density , quantitative ultrasound parameters, and fracture incidence after spinal cord injury:a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;15:180–9. doi: 10.1007/s00198-003-1529-6. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann P, Heilporn A, Schoutens A, Paternot J, Tricot A. Longitudinal study of Calcium and Bone Metabolism in paraplegic patients. Paraplegia. 1977;15(3):147–59. doi: 10.1038/sc.1977.20. [DOI] [PubMed] [Google Scholar]
- 17.Maynard FM. Immobilization hypercalcemia following spinal cord injury. Arch Phys Med Rehabil. 1986;67(1):41–4. [PubMed] [Google Scholar]
- 18.Vaziri ND, Pandiun MR, Segal JL, Winer RL, Eltorai I, Brunnemann S. Vitamin D, Parathormone, and Calcitonin Profiles in Persons With Long-Standing Spinal Cord Injury. Arch Phys Med Rehabil. 1994;75:766–9. [PubMed] [Google Scholar]
- 19.Stewart AF, Adler M, Byers CM, Segre G V, Broadus AE. Calcium Homeostasis in Immobilization:An example of Resorptive Hypercaciuria. N Engl J Med. 1982:1136–40. doi: 10.1056/NEJM198205133061903. [DOI] [PubMed] [Google Scholar]
- 20.Dudley-Javoroski S, Shields RK. Muscle and bone plasticity after spinal cord injury:Review of adaptations to disuse and to electrical muscle stimulation. J Rehabil Res Dev. 2008;45(2):283–96. doi: 10.1682/jrrd.2007.02.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai S, Matsusue Y. Neuronal Regulation of Bone Metabolism and Anabolism:Calcitonin Gene-Related Peptide-, Substance P-, and Tyrosine Hydroxylase-Containing Nerves and the Bone. Microsc Res Tech. 2002;58:61–9. doi: 10.1002/jemt.10119. [DOI] [PubMed] [Google Scholar]
- 22.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, et al. Leptin Regulates Bone Formation via the Sympathetic Nervous System. Cell. 2002;111:305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 23.Dimitri P, Rosen C. The Central Nervous System and Bone Metabolism:An Evolving Story. Calcif Tissue Int. 2017;100(5):476–85. doi: 10.1007/s00223-016-0179-6. [DOI] [PubMed] [Google Scholar]
- 24.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 25.Qin W, Bauman WA, Cardozo CP. Evolving concepts in neurogenic osteoporosis. Curr Osteoporos Rep. 2010;8(4):212–8. doi: 10.1007/s11914-010-0029-9. [DOI] [PubMed] [Google Scholar]
- 26.He J, Zheng X, Jiang L. Autonomic control of bone formation:its clinical relevance. Handbook of Clinical Neurology. 2013:161–71. doi: 10.1016/B978-0-444-53491-0.00014-6. [DOI] [PubMed] [Google Scholar]
- 27.Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a Dense and Intimate Innervation of the Bone Tissue , Including Glutamate-Containing Fibers. Bone. 1999;25(6):623–9. doi: 10.1016/s8756-3282(99)00215-x. [DOI] [PubMed] [Google Scholar]
- 28.Krassioukov A V, Bunge RP, Pucket WR, Bygrave MA. The changes in human spinal sympathetic preganglionic neurons after spinal cord injury. Spinal Cord. 1999;37:6–13. doi: 10.1038/sj.sc.3100718. [DOI] [PubMed] [Google Scholar]
- 29.Chantraine A, Ouwenaller C Van, Hachen HJ, Schinas P. Intra-medullary pressure and intra-osseous phlebography in paraplegia. Paraplegia. 1979;17:391–7. doi: 10.1038/sc.1979.75. [DOI] [PubMed] [Google Scholar]
- 30.Alexander MS, Biering-Sorensen F, Bodner D, Brackett NL, Cardenas D, Charlifue S, et al. International standards to document remaining autonomic function after spinal cord injury. Spinal Cord. 2009;47(1):36–43. doi: 10.1038/sc.2008.121. [DOI] [PubMed] [Google Scholar]
- 31.Maïmoun L, Fattal C, Sultan C. Bone remodeling and calcium homeostasis in patients with spinal cord injury: A review. Metabolism. 2011;60(12):1655–63. doi: 10.1016/j.metabol.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Mechanick JI, Pomerantz F, Flanagan S, Stein A, Wayne GA, Ragnarison KT. Parathyroid Hormone Suppression in Spinal Cord Injury Patients Is Associated With the Degree of Neurologic Impairment and Not the Level of Injury. Arch Phys Med Rehabil. 1997;78:692–6. doi: 10.1016/s0003-9993(97)90075-7. [DOI] [PubMed] [Google Scholar]
- 33.Nemunaitis GA, Mejia M, Nagy JA, Johnson T, Chae J, Roach MJ. A Descriptive Study on Vitamin D Levels in Individuals With Spinal Cord Injury in an Acute Inpatient Rehabilitation Setting. PM R. 2010;2(3):202–8. doi: 10.1016/j.pmrj.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Oleson CV, Patel PH, Wuermser LA. Influence of season, ethnicity, and chronicity on vitamin D deficiency in traumatic spinal cord injury. J Spinal Cord Med. 2010;33(3):202–13. doi: 10.1080/10790268.2010.11689697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flueck JL, Perret C. Vitamin D deficiency in individuals with a spinal cord injury: a literature review. Spinal Cord. 2017;55(5):428–34. doi: 10.1038/sc.2016.155. [DOI] [PubMed] [Google Scholar]
- 36.Bauman WA, Zhong Y, Schwartz E. Vitamin D Deficiency in Veterans With Chronic Spinal Cord Injury. Metabolism. 1995;44(12):1612–6. doi: 10.1016/0026-0495(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 37.Bauman WA, Zhang RL, Morrison N, Spungen AM. Acute suppression of bone turnover with calcium infusion in persons with spinal cord injury. J Spinal Cord Med. 2009;32(4):398–403. doi: 10.1080/10790268.2009.11754393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giangregorio L, Mccartney N. Bone Loss and Muscle Atrophy in Spinal Cord Injury:Epidemiology , Fracture Prediction , and Rehabilitation Strategies. J Spinal Cord Med. 2006;29(5):489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.M Spungen A, Wang J, Pierson RN, Bauman WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol. 2000;88:1310–5. doi: 10.1152/jappl.2000.88.4.1310. [DOI] [PubMed] [Google Scholar]
- 40.Castro MJ, Apple DF, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol. 1999;80:373–8. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PN, Ewins DJ, Fox B, Grundy D, Swain ID. Limb blood flow, cardiac output and quadriceps muscle bulk following spinal cord injury and the effect of training for the Odstock functional electrical stimulation standing system. Paraplegia. 1993;31(5):303–10. doi: 10.1038/sc.1993.53. [DOI] [PubMed] [Google Scholar]
- 42.Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45(4):304–9. doi: 10.1038/sj.sc.3101968. [DOI] [PubMed] [Google Scholar]
- 43.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury - a cross-sectional study. Spinal Cord. 2004;42:711–6. doi: 10.1038/sj.sc.3101652. [DOI] [PubMed] [Google Scholar]
- 44.Gu G, Hentunen TA, Nars M, Harkonen PL, Vaananen HK. Estrogen protects primary osteocytes against glucocorticoid-induced apoptosis. Apoptosis. 2005;10(3):583–95. doi: 10.1007/s10495-005-1893-0. [DOI] [PubMed] [Google Scholar]
- 45.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun 328. 2005;328:688–96. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 46.Riggs BL, Khosla S, Melton LJ. Sex Steroids and the Construction and Conservation of the Adult Skeleton. Endocr Rev. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 47.Huang T, Wang Y, Lai J, Chang C, Lien I. The Hypothalamus-Pituitary-Ovary and Hypothalamus-Pituitary-Thyroid Axes in Spinal Cord-Injured Women. Metabolism. 1996;45(6):718–22. doi: 10.1016/s0026-0495(96)90137-7. [DOI] [PubMed] [Google Scholar]
- 48.NICE. Osteoporosis:assessing the risk of fragility fracture [Internet] 2012. Available from: https://www.nice.org.uk/guidance/cg146/ [PubMed]
- 49.Griffith JF, Genant HK. New advances in imaging osteoporosis and its complications. Endocrine. 2012;42:39–51. doi: 10.1007/s12020-012-9691-2. [DOI] [PubMed] [Google Scholar]
- 50.Genant HK, Engelke K, Fuerst T, Gluer C, Grampp S, Harris ST, et al. Noninvasive Assessment of Bone Mineral and Structure:State of the Art. J Bone Miner Res. 1996;11(6):707–30. doi: 10.1002/jbmr.5650110602. [DOI] [PubMed] [Google Scholar]
- 51.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Osteoporos Int. 2002;359:1929–36. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 52.Bauman WA, Kirshblum S, Cirnigliaro C, Forrest GF, Spungen AM. Underestimation of Bone Loss of the Spine With Posterior-Anterior Dual-Energy X-Ray Absorptiometry in Patients With Spinal Cord Injury. J Spinal Cord Med. 2010;33(3):214–20. doi: 10.1080/10790268.2010.11689698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen SP. The Fallacy of BMD :A Critical Review of the Diagnostic Use of Dual X-ray Absorptiometry. Clin Rheumatol. 2000;19:174–83. doi: 10.1007/s100670050151. [DOI] [PubMed] [Google Scholar]
- 54.Chow YW, Inman C, Pollintine P, Sharp CA, Haddaway MJ, El Masry W, et al. Ultrasound bone densitometry and dual energy X-ray absorptiometry in patients with spinal cord injury:A cross-sectional study. Spinal Cord. 1996;34(12):736–41. doi: 10.1038/sc.1996.134. [DOI] [PubMed] [Google Scholar]
- 55.Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, et al. Relationship between the duration of paralysis and bone structure:a pQCT study of spinal cord injured individuals. Bone. 2004;34:869–80. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Coupaud S, Mclean AN, Allan DB. Role of peripheral quantitative computed tomography in identifying disuse osteoporosis in paraplegia. Skelet Radiol. 2009;38:989–95. doi: 10.1007/s00256-009-0674-1. [DOI] [PubMed] [Google Scholar]
- 57.Frotzler A, Cheikh-Sarraf B, Pourtehrani M, Krebs J, Lippuner K. Long-bone fractures in persons with spinal cord injury. Spinal Cord. 2015;53(9):701–4. doi: 10.1038/sc.2015.74. [DOI] [PubMed] [Google Scholar]
- 58.Nishiyama KK, Shane E. Clinical Imaging of Bone Microarchitecture with HR-pQCT. Curr Osteoporos Rep. 2013;11:147–55. doi: 10.1007/s11914-013-0142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siffert RS, Luo GM, Cowin SC, Kaufman JJ. Dynamic Relationships of Trabecular Bone Density, Architecture, and Strength in a Computational Model of Osteopenia. Bone. 1996;18(2):197–206. doi: 10.1016/8756-3282(95)00446-7. [DOI] [PubMed] [Google Scholar]
- 60.Modlesky CM, Majumdar S, Narasimhan A, Dudley GA. Trabecular Bone Microarchitecture Is Deteriorated in Men With Spinal Cord Injury. J Bone Miner Metab. 2004;19(1):48–55. doi: 10.1359/JBMR.0301208. [DOI] [PubMed] [Google Scholar]
- 61.Giangregorio LM, Gibbs JC, Craven BC. Measuring muscle and bone in individuals with neurologic impairment;lessons learned about participant selection and pQCT scan acquisition and analysis. Osteoporos Int. 2016:2433–46. doi: 10.1007/s00198-016-3572-0. [DOI] [PubMed] [Google Scholar]
- 62.Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36(11):790–6. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]
- 63.Tsuzuku S, Ikegami Y, Yabe K. Bone mineral density differences between paraplegic and quadriplegic patients:a cross-sectional study. Spinal Cord. 1999;37:358–61. doi: 10.1038/sj.sc.3100835. [DOI] [PubMed] [Google Scholar]
- 64.Dauty M, Verbe BP, Maugars Y, Dubois C, Mathe JF. Supralesional and Sublesional Bone Mineral Density in Spinal Cord-Injured Patients. Bone. 2000;27(2):305–9. doi: 10.1016/s8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- 65.Cervinka T, Giangregorio L, Sievanen H, Am C, BC C. Peripheral Quantitative Computed Tomography:Review of Evidence and Recommendations for Image Acquisition, Analysis and Reporting, Among Individuals with Neurological Impairment. J Clin Densitom. 2018;21(4):563–82. doi: 10.1016/j.jocd.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 66.Morse LR, Biering-Soerensen F, Carbone LD, Cervinka T, Cirnigliaro CM, Johnston TE, et al. Bone Mineral Density Testing in Spinal Cord Injury:2019 ISCD Official Position. J Clin Densitom. 2019;22(4):554–66. doi: 10.1016/j.jocd.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 67.Bauman WA, Cirnigliaro CM, Fountaine MF La, Martinez L, Kirshblum SC, Spungen AM. Zoledronic acid administration failed to prevent bone loss at the knee in persons with acute spinal cord injury:an observational cohort study. J Bone Miner Metab. 2015;33:410–21. doi: 10.1007/s00774-014-0602-x. [DOI] [PubMed] [Google Scholar]
- 68.Cirnigliaro CM, Myslinski MJ, Asselin P, Hobson JC, Specht A, La Fountaine MF, et al. Progressive Sublesional Bone Loss Extends into the Second Decade After Spinal Cord Injury. J Clin Densitom. 2019;22(2):185–94. doi: 10.1016/j.jocd.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Bakkum AJT, Janssen TWJ, Rolf MP, Roos JC, Burcksen J, Knol DL, et al. A reliable method for measuring proximal tibia and distal femur bone mineral density using dual-energy X-ray absorptiometry. Med Eng Phys. 2014;36(3):387–90. doi: 10.1016/j.medengphy.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Trbovich M, Mack D, Bruder JM. Osteoporosis in Veterans with Spinal Cord Injury:an Overview of Pathophysiology, Diagnosis, and Treatments. Clin Rev Bone Miner Metab. 2019;17(2):94–108. [Google Scholar]
- 71.Cirnigliaro CM, Myslinski MJ, La Fountaine MF, Kirshblum SC, Forrest GF, Bauman WA. Bone loss at the distal femur and proximal tibia in persons with spinal cord injury:imaging approaches, risk of fracture, and potential treatment options. Osteoporos Int. 2017;28(3):747–65. doi: 10.1007/s00198-016-3798-x. [DOI] [PubMed] [Google Scholar]
- 72.McPherson JG, Edwards WB, Prasad A, Troy KL, Griffith JW, Schnitzer TJ. Dual energy X-ray absorptiometry of the knee in spinal cord injury:Methodology and correlation with quantitative computed tomography. Spinal Cord. 2014;52(11):821–5. doi: 10.1038/sc.2014.122. [DOI] [PubMed] [Google Scholar]
- 73.Lala D, Craven BC, Thabane L, Papaioannou A, Adachi JD, Popovic MR, et al. Exploring the determinants of fracture risk among individuals with spinal cord injury. Osteoporos Int. 2014;25(1):177–85. doi: 10.1007/s00198-013-2419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coupaud S, Mclean AN, Purcell M, Fraser MH, Allan DB. Decreases in bone mineral density at cortical and trabecular sites in the tibia and femur during the first year of spinal cord injury. Bone. 2015;74(367):684–93. doi: 10.1016/j.bone.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Frotzler A, Berger M, Knecht H, Eser P. Bone steady-state is established at reduced bone strength after spinal cord injury:A longitudinal study using peripheral quantitative computed tomography (pQCT) Bone. 2008;43(3):549–55. doi: 10.1016/j.bone.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 76.Coupaud S, Mclean AN, Lloyd S, Allan DB, Mclean AN, Lloyd S, et al. Predicting patient-specific rates of bone loss at fracture-prone sites after spinal cord injury. Disabil Rehabil. 2012 May;8288:2242–2250. doi: 10.3109/09638288.2012.681831. [DOI] [PubMed] [Google Scholar]
- 77.de Bruin ED, Dietz V, Dambacher MA, Stüssi E. Longitudinal changes in bone in men with spinal cord injury. Clin Rehabil. 2000;14(2):145–52. doi: 10.1191/026921500670532165. [DOI] [PubMed] [Google Scholar]
- 78.Bauman WA, Spungen AM, Wang J, Pierson RN, Schwartz E. International Original Article Continuous Loss of Bone During Chronic Immobilization:A Monozygotic Twin Study. Osteoporos Int. 1999;10:123–7. doi: 10.1007/s001980050206. [DOI] [PubMed] [Google Scholar]
- 79.Szollar SM, Martin EME, Parthemore JG, Sartoris DJ, Deftos LJ. Densitometric patterns of spinal cord injury associated bone loss. Spinal Cord. 1997;35(6):374–82. doi: 10.1038/sj.sc.3100394. [DOI] [PubMed] [Google Scholar]
- 80.Rittweger J, Goosey-Tolfrey VL, Cointry G, Ferretti JL. Structural analysis of the human tibia in men with spinal cord injury by tomographic (pQCT) serial scans. Bone. 2010;47(3):511–8. doi: 10.1016/j.bone.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 81.Sievanen H, Koskue V, Rauhio A, Kannus P, Heinonen ARI, VUORI I. Peripheral Quantitative Computed Tomography in Human Long Bones:Evaluation of In Vitro and In Vivo Precision. J Bone Miner Res. 1998;13(5):871–82. doi: 10.1359/jbmr.1998.13.5.871. [DOI] [PubMed] [Google Scholar]
- 82.Dionyssiotis Y, Trovas G, Galanos A, Raptou P, Papaioannou N, Papagelopoulos P, et al. Bone loss and mechanical properties of tibia in spinal cord injured men. J Musculoskelet Neuronal Interact. 2007;7(1):62–8. [PubMed] [Google Scholar]
- 83.Kaya K, Aybay C, Ozel S, Kutay N, Gokkaya O. Evaluation of Bone Mineral Density in Patients With Spinal Cord Injury. J Spinal Cord Med. 2006;29:396–401. doi: 10.1080/10790268.2006.11753888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kiratli BJ, Smith AE, Nauenberg T, Kallfelz CF, Perkash I. Bone mineral and geometric changes through the femur with immobilization due to spinal cord injury. J Rehabil Res Dev. 2000;37(2):225–33. [PubMed] [Google Scholar]
- 85.Frey-Rindova P, Bruin ED De, Stu E. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38:26–32. doi: 10.1038/sj.sc.3100905. [DOI] [PubMed] [Google Scholar]
- 86.Morse LR, Sudhakar S, Danilack V, Tun C, Lazzari A, Gagnon DR, et al. Association between sclerostin and bone density in chronic spinal cord injury. J Bone Miner Res. 2012;27(2):352–9. doi: 10.1002/jbmr.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Biering-Serensen F, Bohr H, Schaadt O. Bone mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Paraplegia. 1988;26(5):293–301. doi: 10.1038/sc.1988.44. [DOI] [PubMed] [Google Scholar]
- 88.Gaspar AP, Lazaretti-Castro M, Brandão CMA. Bone mineral density in spinal cord injury:An evaluation of the distal femur. J Osteoporos. 2012;2012:1–7. doi: 10.1155/2012/519754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rittweger J, Gerrits K, Altenburg T, Reeves N, Maganaris CN, de Haan A. Bone adaptation to altered loading after spinal cord injury:A study of bone and muscle strength. J Musculoskelet Neuronal Interact. 2006;6(3):269–76. [PubMed] [Google Scholar]
- 90.Sabo D, Blaich S, Wenz W, Hohmann M, Loew M. Osteoporosis in patients with paralysis after spinal cord injury. Arch Orthop Trauma Surg. 2001;121:75–8. doi: 10.1007/s004020000162. [DOI] [PubMed] [Google Scholar]
- 91.Wood DE, Dunkerley AL, Tromans AM. Results from bone mineral density scans in twenty-two complete lesion paraplegics. Spinal Cord. 2001;39:145–8. doi: 10.1038/sj.sc.3101125. [DOI] [PubMed] [Google Scholar]
- 92.Karapolat I, Karapolat HU, Kirazli Y, Capaci K, Akkoc Y, Kumanlioglu K. Longitudinal study of bone loss in chronic spinal cord injury patients. J Phys Ther Sci. 2015;27(5):1429–33. doi: 10.1589/jpts.27.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bauman WA, Schwartz E, Song ISY, Kirshblum S, Cirnigliaro C, Morrison N, et al. Dual-energy X-ray absorptiometry overestimates bone mineral density of the lumbar spine in persons with spinal cord injury. Spinal Cord. 2009;47(8):628–33. doi: 10.1038/sc.2008.169. [DOI] [PubMed] [Google Scholar]
- 94.Garland DE, Adkins RH, Stewart CA, Ashford R, Vigil D. Regional osteoporosis in women who have a complete spinal cord injury. J Bone Jt Surg - Ser A. 2001;83(8):1195–200. doi: 10.2106/00004623-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 95.Demirel G, Yilmaz H, Paker N, Onel S. Osteoporosis after spinal cord injury. Spinal Cord. 1998;36:822–5. doi: 10.1038/sj.sc.3100704. [DOI] [PubMed] [Google Scholar]
- 96.Eser P, Frotzler A, Zehnder Y, Schiessl H, Denoth J. Assessment of anthropometric, systemic, and lifestyle factors influencing bone status in the legs of spinal cord injured individuals. Osteoporos Int. 2005;16(1):26–34. doi: 10.1007/s00198-004-1638-x. [DOI] [PubMed] [Google Scholar]
- 97.Löfvenmark I, Werhagen L, Norrbrink C. Spasticity and bone density after a spinal cord injury. J Rehabil Med. 2009;41:1080–4. doi: 10.2340/16501977-0469. [DOI] [PubMed] [Google Scholar]
- 98.Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–7. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- 99.Gifre L, Vidal J, Carrasco J, Portell E, Puig J, Monegal A, et al. Incidence of skeletal fractures after traumatic spinal cord injury:a 10-year follow-up study. Clin Rehabil. 2014;28(4):361–9. [Google Scholar]
- 100.Freehafer AA, Hazel CM, Becker CL. Lower extremity fractures in Patients with Spinal Cord Injury. Paraplegia. 1981;19:367–72. doi: 10.1038/sc.1981.69. [DOI] [PubMed] [Google Scholar]
- 101.Freehafer AA. Limb fractures in patients with spinal cord injury. Arch Phys Med Rehabil. 1995;76(1):823–7. doi: 10.1016/s0003-9993(95)80546-x. [DOI] [PubMed] [Google Scholar]
- 102.Ingram RR, Suman RK, Freeman PA. Lower Limb Fractures in the Chronic Spinal Cord Injured Patient. Paraplegia. 1989;27:133–9. doi: 10.1038/sc.1989.20. [DOI] [PubMed] [Google Scholar]
- 103.Hartkopp A, Murphy RJL, Mohr T, Kjcq M, Biering-s F. Bone Fracture During Electrical Stimulation of the Quadriceps in a Spinal Cord Injured Subject. Arch Phys Med Rehabil. 1998 Sep;79:1133–6. doi: 10.1016/s0003-9993(98)90184-8. [DOI] [PubMed] [Google Scholar]
- 104.Filippo TRM, De Carvalho MCL, Carvalho LB, De Souza DR, Imamura M, Battistella LR. Proximal tibia fracture in a patient with incomplete spinal cord injury associated with robotic treadmill training. Spinal Cord. 2015;53(12):875–6. doi: 10.1038/sc.2015.27. [DOI] [PubMed] [Google Scholar]
- 105.Morse LR, Battaglino RA, Stolzmann KL, Hallett LD, Waddimba A, Gagnon D, et al. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int. 2009;20(3):385–92. doi: 10.1007/s00198-008-0671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bethel M, Weaver FM, Bailey L, Miskevics S, Svircev JN, Burns SP, et al. Risk factors for osteoporotic fractures in persons with spinal cord injuries and disorders. Osteoporos Int. 2016;27(10):3011–21. doi: 10.1007/s00198-016-3627-2. [DOI] [PubMed] [Google Scholar]
- 107.Carbone LD, Chin AS, Burns SP, Svircev JN, Hoenig H, Heggeness M, et al. Morbidity following lower extremity fractures in men with spinal cord injury. Osteoporos Int. 2013;24:2261–7. doi: 10.1007/s00198-013-2295-8. [DOI] [PubMed] [Google Scholar]
- 108.Shields R K, Dudley-Javoroski S. Musculoskeletal Adaptations in Chronic Spinal Cord Injury:Effects of Long-term Soleus Electrical Stimulation Training. Neurorehabil Neural Repair. 2007;21(2):169–79. doi: 10.1177/1545968306293447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dudley-Javoroski S, Saha P, Liang G, Li C, Gao Z, Shields RK. High dose compressive loads attenuate bone mineral loss in humans with spinal cord injury. Osteoporos Int. 2012;23(9):2335–46. doi: 10.1007/s00198-011-1879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shields RK, Dudley-Javoroski S, Frey Law LA. Electrically Induced Muscle Contractions Influence Bone Density Decline After Spinal Cord Injury. Spine (Phila Pa 1976) 2006;31(5):548–53. doi: 10.1097/01.brs.0000201303.49308.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bélanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation:Can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil. 2000;81(8):1090–8. doi: 10.1053/apmr.2000.7170. [DOI] [PubMed] [Google Scholar]
- 112.Clark JM, Jelbart M, Rischbieth H, Strayer J, Chatterton B, Schultz C, et al. Physiological effects of lower extremity functional electrical stimulation in early spinal cord injury:lack of efficacy to prevent bone loss. Spinal Cord. 2007;45:78–85. doi: 10.1038/sj.sc.3101929. [DOI] [PubMed] [Google Scholar]
- 113.Eser P, De Bruin ED, Telley I, Lechner HE, Knecht H, Stüssi E. Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord-injured patients. Eur J Clin Invest. 2003;33(5):412–9. doi: 10.1046/j.1365-2362.2003.01156.x. [DOI] [PubMed] [Google Scholar]
- 114.Mohr T, Pødenphant J, Biering-Sørensen F, Galbo H, Thamsborg G, Kjær M. Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int. 1997;61(1):22–5. doi: 10.1007/s002239900286. [DOI] [PubMed] [Google Scholar]
- 115.Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, Donaldson N de N, et al. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone. 2008;43(1):169–76. doi: 10.1016/j.bone.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 116.Gibbons RS, Beaupre GS, Kazakia GJ. FES-rowing attenuates bone loss following spinal cord injury as assessed by HR-pQCT. Spinal Cord Ser Cases. 2016;2(1):15041. doi: 10.1038/scsandc.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lai C, Chang WH, Chan WP, Shen L, Chen JJ, Chen S. Effects of functional electrical stimulation cycling exercise on bone mineral density loss in the early stages of spinal cord injury. J Rehabil Med. 2010;42:150–4. doi: 10.2340/16501977-0499. [DOI] [PubMed] [Google Scholar]
- 118.Hjeltnes N, Aksnes AK, Birkeland KI, Johansen J, Lannem A, Wallberg-Henriksson H. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol. 1997;273:R1072–9. doi: 10.1152/ajpregu.1997.273.3.R1072. [DOI] [PubMed] [Google Scholar]
- 119.Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol. 2009;19(4):614–22. doi: 10.1016/j.jelekin.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 120.Dudley GA, Castro MJ, Rogers S, Apple DF, Jr, Apple DF, Apple DF., Jr A simple means of increasing muscle size after spinal cord injury:A pilot study. Eur J Appl Physiol Occup Physiol. 1999;80:394–6. doi: 10.1007/s004210050609. [DOI] [PubMed] [Google Scholar]
- 121.Crameri RM, Cooper P, Sinclair PJ, Bryant G, Weston A. Effect of load during electrical stimulation training in spinal cord injury. Muscle and Nerve. 2004;29(1):104–11. doi: 10.1002/mus.10522. [DOI] [PubMed] [Google Scholar]
- 122.Shields R K, Dudley-Javoroski S. Musculoskeletal Plasticity After Acute Spinal Cord Injury:Effects of Long-Term Neuromuscular Electrical Stimulation Training. J Neurophysiol. 2006;95(1):1–23. doi: 10.1152/jn.01181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sloan KE, Bremner LA, Byrne J, Day RE, Scull ER. Musculoskeletal effects of an electrical stimulation induced cycling programme in the spinal injured. Paraplegia. 1994;32(6):407–15. doi: 10.1038/sc.1994.67. [DOI] [PubMed] [Google Scholar]
- 124.Astorino TA, Harness ET, Witzke KA. Effect of chronic activity-based therapy on bone mineral density and bone turnover in persons with spinal cord injury. Eur J Appl Physiol. 2013;113(12):3027–37. doi: 10.1007/s00421-013-2738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bloomfield SA, Mysiw WJ, Jackson RD. Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone. 1996;19(1):61–8. doi: 10.1016/8756-3282(96)00109-3. [DOI] [PubMed] [Google Scholar]
- 126.Frost HM. From Wolff 's Law to the Utah Paradigm :Insights About Bone Physiology and Its Clinical Applications. Anat Rec. 2001 Feb;262:398–419. doi: 10.1002/ar.1049. [DOI] [PubMed] [Google Scholar]
- 127.Chen SC, Lai CH, Chan WP, Huang MH, Tsai HW, Chen JJJ. Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil Rehabil. 2005;27(22):1337–41. doi: 10.1080/09638280500164032. [DOI] [PubMed] [Google Scholar]
- 128.Rodgers MM, Glaser RM, Figoni SE, Hooker SP, Ezenwa BN, Collins SR, et al. Musculoskeletal responses of spinal cord injured individuals to functional neuromuscular stimulation-induced knee extension exercise training. J Rehabil Res Dev. 1991;28(4):19–26. doi: 10.1682/jrrd.1991.10.0019. [DOI] [PubMed] [Google Scholar]
- 129.Gibbons RS, Mccarthy ID, Gall A, Stock CG, Shippen J, Andrews BJ. Can FES-rowing mediate bone mineral density in SCI:A pilot study. Spinal Cord. 2014;52:S4–5. doi: 10.1038/sc.2014.112. [DOI] [PubMed] [Google Scholar]
- 130.Pacy PJ, Hesp R, Halliday DA, Katz D, Cameron G, Reeve J. Muscle and bone in paraplegic patients, and the effect of functional electrical stimulation. Clin Sci. 1988;75(5):481–7. doi: 10.1042/cs0750481. [DOI] [PubMed] [Google Scholar]
- 131.Johnston TE, Marino RJ, Oleson CV, Schmidt-Read M, Leiby BE, Sendecki J, et al. Musculoskeletal Effects of 2 Functional Electrical Stimulation Cycling Paradigms Conducted at Different Cadences for People With Spinal Cord Injury: A Pilot Study. Arch Phys Med Rehabil. 2016;97(9):1413–22. doi: 10.1016/j.apmr.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 132.Luepongsak N, Amin S, Krebs DE, McGibbon CA, Felson D. The contribution of type of daily activity to loading across the hip and knee joints in the elderly. Osteoarthr Cartil. 2002;10(5):353–9. doi: 10.1053/joca.2000.0511. [DOI] [PubMed] [Google Scholar]
- 133.Yoshioka S, Nagano A, Hay DC, Fukashiro S. The minimum required muscle force for a sit-to-stand task. J Biomech. 2012;45(4):699–705. doi: 10.1016/j.jbiomech.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 134.Dudley-Javoroski S, Shields RK. Active-resisted stance modulates regional bone mineral density in humans with spinal cord injury. J Spinal Cord Med. 2013;36(3):191–9. doi: 10.1179/2045772313Y.0000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dudely-Javoroski S, Shields RK. Asymmetric bone adaptations to soleus mechanical loading after spinal cord injury S. J Musculoskelet Neuronal Interact. 2008;8(3):227–38. [PMC free article] [PubMed] [Google Scholar]
- 136.Kunkel CF, Scremin AM, Eisenberg B, Garcia JF, Roberts S, Martinez S. Effect of “standing”on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil. 1993;74(1):73–8. [PubMed] [Google Scholar]
- 137.Frey-Rindova P, de Bruin ED, Stussi E, Dambacher M, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38:26–32. doi: 10.1038/sj.sc.3100905. [DOI] [PubMed] [Google Scholar]
- 138.Goktepe AS, Tugcu I, Yilmaz B, Alaca R, Gunduz S. Does Standing Protect Bone Density in Patients With Chronic Spinal Cord Injury ? J Spinal Cord Med. 2008;31:197–201. doi: 10.1080/10790268.2008.11760712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ben M, Harvey L, Denis S, Glinsky J, Goehl G, Chee S, et al. Does 12 weeks of regular standing prevent loss of ankle mobility and bone mineral density in people with recent spinal cord injuries? Aust J Physiother. 2005;51(4):251–6. doi: 10.1016/s0004-9514(05)70006-4. [DOI] [PubMed] [Google Scholar]
- 140.Karelis A, Carvalho L, Castillo M, Gagnon D, Aubertin-Leheudre M. Effect on body composition and bone mineral density of walking with a robotic exoskeleton in adults with chronic spinal cord injury. J Rehabil Med. 2017;49(1):84–7. doi: 10.2340/16501977-2173. [DOI] [PubMed] [Google Scholar]
- 141.Thoumie P, Claire G Le, Beillot J, Dassonville J, Chevalier T, Perrouin-verbes B, et al. Restoration of functional gait in paraplegic patients with the RGO-II hybrid orthosis. A multicenter controlled study. II:Physiological evaluation. Paraplegia. 1995;33:654–9. doi: 10.1038/sc.1995.137. [DOI] [PubMed] [Google Scholar]
- 142.Needham-shropshire B, Broton JG, Klose KJ, Lebwohi N, Guest RS, Jacobs PL. Evaluation of a Training Program for Persons With SCI Paraplegia Using the Parastep®l Ambulation System:Part 3. Lack of Effect on Bone Mineral Density. Arch Phys Med Rehabil. 1997;78:799–803. doi: 10.1016/s0003-9993(97)90190-8. [DOI] [PubMed] [Google Scholar]
- 143.Klose KJ, Jacobs PL, Broton J, Guest RS, Shropshire BMN, Lebwohl N, et al. Evaluation of a Training Program for Persons With SCI Paraplegia Using the Parastep ®l Ambulation System:Part 1. Ambulation Performance and Anthropometric Measures. Arch Phys Med Rehabil. 1997;78:789–93. doi: 10.1016/s0003-9993(97)90188-x. [DOI] [PubMed] [Google Scholar]
- 144.Giangregorio L, Hicks A, Webber C, Phillips SM, Craven BC, Bugaresti J, et al. Body weight supported treadmill training in acute spinal cord injury:impact on muscle and bone. Spinal Cord. 2005;43:649–57. doi: 10.1038/sj.sc.3101774. [DOI] [PubMed] [Google Scholar]
- 145.Giangregorio LM, Webber CE, Phillips SM, Hicks AL, Craven C, Bugaresti JM, et al. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nutr Metab. 2006;31:283–91. doi: 10.1139/h05-036. [DOI] [PubMed] [Google Scholar]
- 146.de Bruin E, Frey-Rindova P, Herzog RE, Dietz V, Dambacher MA, Stüssi E. Changes in tibia bending stiffness two years after spinal cord injury. Arch Phys Med Rehabil. 1999;80:214–20. doi: 10.1016/s0003-9993(99)90124-7. [DOI] [PubMed] [Google Scholar]
- 147.Coupaud S, Jack LP, Hunt KJ, Allan DB. Muscle and bone adaptations after treadmill training in incomplete Spinal Cord Injury:a case study using peripheral Quantitative Computed Tomography. J Musculoskelet Neuronal Interact. 2009;9(4):288–97. [PubMed] [Google Scholar]
- 148.Alekna V, Tamulaitiene M, Sinevicius T, Juocevicius A. Effect of weight-bearing activities on bone mineral density in spinal cord injured patients during the period of the first two years. Spinal Cord. 2008;46:727–32. doi: 10.1038/sc.2008.36. [DOI] [PubMed] [Google Scholar]
- 149.Goemaere S, Laere M Van, Neve P De, Kaufman JM. Bone Mineral Status in Paraplegic Patients Who Do or Do Not Perform Standing. Osteoporos Int. 1994;4:138–43. doi: 10.1007/BF01623058. [DOI] [PubMed] [Google Scholar]
- 150.Hsieh YF, Robling AG, Ambrosius WT, Burr DB, Turner CH. Mechanical loading of diaphyseal bone in vivo:The strain threshold for an osteogenic response varies with location. J Bone Miner Res. 2001;16(12):2291–7. doi: 10.1359/jbmr.2001.16.12.2291. [DOI] [PubMed] [Google Scholar]
- 151.Carvalho DCL, Garlipp CR, Bottini P V, Afaz SH, Moda MA, Cliquet A., Jr Effect of treadmill gait on bone markers and bone mineral density of quadriplegic subjects. Brazilian J Med Biol Res. 2006;39:1357–63. doi: 10.1590/s0100-879x2006001000012. [DOI] [PubMed] [Google Scholar]
- 152.Biering-Sorensen F, Hansen B, Lee B. Non-pharmacological treatment and prevention of bone loss after spinal cord injury:a systematic review. Spinal Cord. 2009;47:508–18. doi: 10.1038/sc.2008.177. [DOI] [PubMed] [Google Scholar]
- 153.Yang P, Sanno M, Ganse B, Koy T, Bruggemann G, Muller LP, et al. Torsion and Antero-Posterior Bending in the In Vivo Human Tibia Loading Regimes during Walking and Running. PLoS One. 2014;9(4):1–12. doi: 10.1371/journal.pone.0094525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Galea MP. Spinal cord injury and physical activity:preservation of the body. Spinal Cord. 2012;50(5):344–51. doi: 10.1038/sc.2011.149. [DOI] [PubMed] [Google Scholar]
- 155.Nolte PA, Klein-nulend J, Albers GHR, Marti RK, Semeins CM, Burger EH. Low-intensity ultrasound stimulates endochondral ossification in vitro. J Orthop Res. 2001;21:301–7. doi: 10.1016/S0736-0266(00)00027-9. [DOI] [PubMed] [Google Scholar]
- 156.Naruse K, Mikuni-takagaki Y, Azuma Y, Ito M, Oota T, Kameyama K, et al. Anabolic Response of Mouse Bone-Marrow-Derived Stromal Cell Clone ST2 Cells to Low-Intensity Pulsed Ultrasound. Biochem Biophys Res Commun. 2000;268:216–20. doi: 10.1006/bbrc.2000.2094. [DOI] [PubMed] [Google Scholar]
- 157.Warden SJ, Bennell KL, Matthews B, Brown DJ, McMeeken JM, Wark JD. Efficacy of low-intensity pulsed ultrasound in the prevention of osteoporosis following spinal cord injury. Bone. 2001;29(5):431–6. doi: 10.1016/s8756-3282(01)00599-3. [DOI] [PubMed] [Google Scholar]
- 158.Dudley-Javoroski S, Petrie MA, Mchenry CL, Amelon RE, Saha PK, Shields RK. Bone architecture adaptations after spinal cord injury:impact of long-term vibration of a constrained lower limb. Osteooporosis Int. 2016;27:1149–60. doi: 10.1007/s00198-015-3326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wuermser L, Beck LA, Lamb JL, Atkinson EJ, Amin S. The effect of low-magnitude whole body vibration on bone density and microstructure in men and women with chronic motor complete paraplegia. J Spinal Cord Med. 2015;38(2):178–86. doi: 10.1179/2045772313Y.0000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Davis R, Sanborn C, Nichols D, Bazett-jones DM, Dugan EL. The Effects of Whole Body Vibration on Bone Mineral Density for a Person With a Spinal Cord Injury:A Case Study. Adapt Phys Act Q. 2010;27:60–72. doi: 10.1123/apaq.27.1.60. [DOI] [PubMed] [Google Scholar]
- 161.Schriefer JL, Warden SJ, Saxon LK, Robling AG, Turner CH. Cellular accommodation and the response of bone to mechanical loading. J Biomech. 2005;38:1838–45. doi: 10.1016/j.jbiomech.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 162.Noble BS, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, et al. Mechanical loading:biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. J Physiol Cell Physiol. 2003;284:934–43. doi: 10.1152/ajpcell.00234.2002. [DOI] [PubMed] [Google Scholar]
- 163.Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K, et al. Adaptive response of human tendon to paralysis. Muscle and Nerve. 2006;33(1):85–92. doi: 10.1002/mus.20441. [DOI] [PubMed] [Google Scholar]
- 164.Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, et al. The Effects of Strontium Ranelate on the Risk of Vertebral Fracture in Women with Postmenopausal Osteoporosis. N Engl J Med. 2004;350(5):459–68. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 165.Cummings SR, Martin JS, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 166.Avioli L V. SERM drugs for the prevention of osteoporosis. Trends Endocrinol Metab. 1999;10(8):317–9. doi: 10.1016/s1043-2760(99)00176-9. [DOI] [PubMed] [Google Scholar]
- 167.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, et al. Effect of Oral Alendronate on Bone Mineral Density and the Incidence of Fractures in Postmenopausal Osteoporosis. N Engl J Med. 1995;22(333):1437–43. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 168.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-Yearly Zoledronic Acid for Treatment of Postmenopausal Osteoporosis. N Engl J Med. 2007;356(18):1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 169.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of Risedronate on the Risk of Hip Fracture in Elderly Women. N Engl J Med. 2001;344(5):333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 170.Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the Treatment of Osteoporosis in Men. N Engl J Med. 2000;343(9):604–10. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 171.Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women:a randomized trial. J Bone Miner Res. 2000;15(6):1006–13. doi: 10.1359/jbmr.2000.15.6.1006. [DOI] [PubMed] [Google Scholar]
- 172.Watts NB, Diab DL. Long-Term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555–65. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 173.de Brito CM, Battistella LR, Saito ET, Sakamoto H. Effect of alendronate on bone mineral density in spinal cord injury patients:a pilot study. Spinal Cord. 2005;43:341–8. doi: 10.1038/sj.sc.3101725. [DOI] [PubMed] [Google Scholar]
- 174.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18(8):1023–31. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 175.Shapiro J, Smith B, Beck T, Ballard P, Dapthary M, Brintzenhofeszoc K, et al. Treatment with Zoledronic Acid Ameliorates Negative Geometric Changes in the Proximal Femur following Acute Spinal Cord Injury. Calcif Tissue Int. 2007;80:316–22. doi: 10.1007/s00223-007-9012-6. [DOI] [PubMed] [Google Scholar]
- 176.Bauman WA, Wecht JM, Kirshblum S, Spungen AM, Morrison N, Cirnigliaro C, et al. Effect of pamidronate administration on bone in patients with acute spinal cord injury. J Rehabil Res Dev. 2005;42(3):305–14. doi: 10.1682/jrrd.2004.05.0062. [DOI] [PubMed] [Google Scholar]
- 177.Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D. Intravenous Pamidronate Attenuates Bone Density Loss After Acute Spinal Cord Injury. Arch Phys Med Rehabil. 1999;80:243–51. doi: 10.1016/s0003-9993(99)90133-8. [DOI] [PubMed] [Google Scholar]
- 178.Gilchrist NL, Frampton CM, Acland RH, Nicholls MG, March RL, Maguire P, et al. Alendronate Prevents Bone Loss in Patients with Acute Spinal Cord Injury:A Randomized , Double-Blind , Placebo-Controlled Study. J Clin Endocrinol Metab. 2007;92(4):1385–90. doi: 10.1210/jc.2006-2013. [DOI] [PubMed] [Google Scholar]
- 179.Bubbear JS, Gall A, Middleton FRI, Ferguson-Pell M, Swaminathan R, Keen RW. Early treatment with zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporos Int. 2011;22:271–9. doi: 10.1007/s00198-010-1221-6. [DOI] [PubMed] [Google Scholar]
- 180.Minaire P, Goedert G, Pilonchery G. Effects of disodium dichloromethylene diphosphonate on bone loss in paraplegic patients. J Clin Invest. 1981;68(4):1086–92. doi: 10.1172/JCI110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Eser P, Frotzler A, Zehnder Y, Denoth J. Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil. 2005;86(3):498–504. doi: 10.1016/j.apmr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 182.Sniger W, Garshick E. Alendronate Increases Bone Density in Chronic Spinal Cord Injury:A Case Report. Arch Phys Med Rehabil. 2002;83(1):139–40. doi: 10.1053/apmr.2002.26828. [DOI] [PubMed] [Google Scholar]
- 183.Bauman WA, Spungen AM, Morrison N, Zhang R, Schwartz E. Effect of a vitamin D analog on leg bone mineral density in patients with chronic spinal cord injury. J Rehabil Res Dev. 2005;42(5):625–33. doi: 10.1682/jrrd.2004.11.0145. [DOI] [PubMed] [Google Scholar]
- 184.Zehnder Y, Risi S, Michel D, Knecht H, Perrelet R, Kraenzlin M, et al. Prevention of Bone Loss in Paraplegics Over 2 Years With Alendronate. J Bone Miner Res. 2004;19(6):1067–74. doi: 10.1359/JBMR.040313. [DOI] [PubMed] [Google Scholar]
- 185.Haider IT, Simonian N, Saini AS, Leung FM, Edwards WB, Schnitzer TJ. Open-label clinical trial of alendronate after teriparatide therapy in people with spinal cord injury and low bone mineral density. Spinal Cord. 2019;57:832–842. doi: 10.1038/s41393-019-0303-3. [DOI] [PubMed] [Google Scholar]
- 186.Mechanick JI, Liu K, Nierman DM, Stein A. Effect of a Convenient Single 90-mg Pamidronate Dose on Biochemical Markers of Bone Metabolism in Patients With Acute Spinal Cord Injury. J Spinal Cord Med. 2006;29(4):406–12. doi: 10.1080/10790268.2006.11753890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pearson EG, Vance PW, Leslie WD, Ludwig S. Cyclical Etidronate:Its Effect on Bone Density in Patients With Acute Spinal Cord Injury. Arch Phys Med Rehabil. 1997 Mar;78:269–72. doi: 10.1016/s0003-9993(97)90032-0. [DOI] [PubMed] [Google Scholar]
- 188.Bryson JE, Gourlay ML. Bisphosphonate Use in Acute and Chronic Spinal Cord Injury:A Systematic Review. J Spinal Cord Med. 2009;32(3):215–25. doi: 10.1080/10790268.2009.11760776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate-Associated Osteonecrosis of the Jaw:Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–91. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 190.Giusti A, Hamdy NAT, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy A systematic review of case / case series studies. Bone. 2010;47(2):169–80. doi: 10.1016/j.bone.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 191.Gifre L, Vidal J, Carrasco JL, Muxi A, Portell E, Monegal A, et al. Denosumab increases sublesional bone mass in osteoporotic individuals with recent spinal cord injury. Osteoporos Int. 2016;27(1):405–10. doi: 10.1007/s00198-015-3333-5. [DOI] [PubMed] [Google Scholar]
- 192.Gordon KE, Wald MJ, Schnitzer TJ. Effect of Parathyroid Hormone Combined With Gait Training on Bone Density and Bone Architecture in People With Chronic Spinal Cord Injury. PMRJ. 2013;5(8):663–71. doi: 10.1016/j.pmrj.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 193.Zhang J, Gao R, Cao P, Yuan W. Additive effects of antiresorptive agents and exercise on lumbar spine bone mineral density in adults with low bone mass:a meta-analysis. Osteoporos Int. 2014;25:1585–94. doi: 10.1007/s00198-014-2644-2. [DOI] [PubMed] [Google Scholar]
- 194.Zhao R, Xu Z, Zhao M. Antiresorptive Agents Increase the Effects of Exercise on Preventing Postmenopausal Bone Loss in Women:A Meta-Analysis. PLoS One. 2015;10(1):1–15. doi: 10.1371/journal.pone.0116729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ireland A, Rittweger J. Exercise for osteoporosis:how to navigate between overeagerness and defeatism A. J Musculoskelet Neuronal Interact. 2017;17(3):155–61. [PMC free article] [PubMed] [Google Scholar]
- 196.Maïmoun L, Fattal C, Micallef JP, Peruchon E, Rabischong P. Bone loss in spinal cord-injured patients:From physiopathology to therapy. Spinal Cord. 2006;44(4):203–10. doi: 10.1038/sj.sc.3101832. [DOI] [PubMed] [Google Scholar]
- 197.Morse LR, Troy KL, Fang Y, Nguyen N, Battaglino R, Goldstein RF, et al. Combination Therapy With Zoledronic Acid and FES-Row Training Mitigates Bone Loss in Paralyzed Legs:Results of a Randomized Comparative Clinical Trial. JBMR Plus. 2019:1–11. doi: 10.1002/jbm4.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Edwards WB, Simonian N, Haider IT, Anschel AS, Chen D, Gordon KE, et al. Effects of Teriparatide and vibration on bone mass and bone strength in people with bone loss and spinal cord injury:a randomized, controlled trial. JBMR. 2018;33(10):1729–40. doi: 10.1002/jbmr.3525. [DOI] [PubMed] [Google Scholar]


