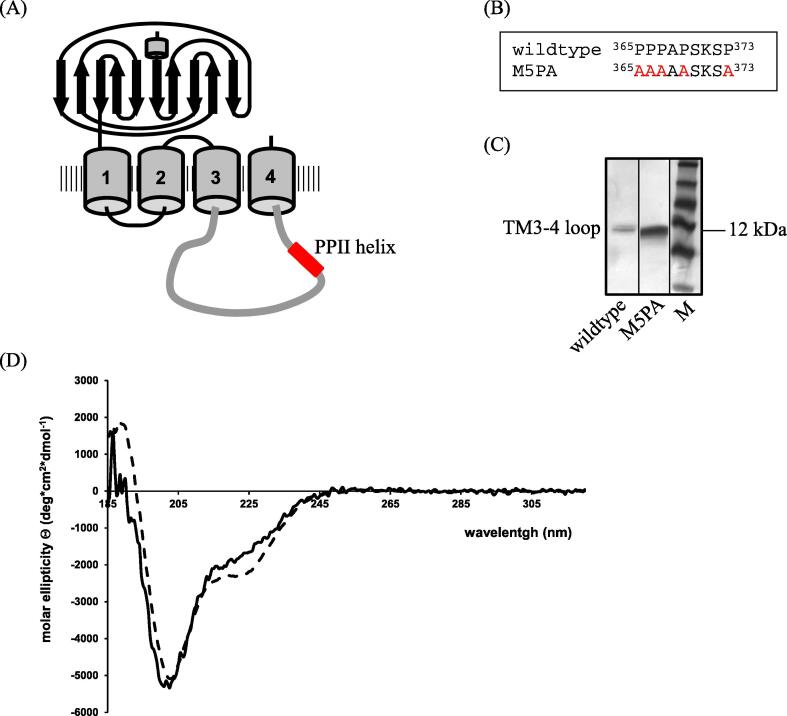
Fig. 1.
Localization and characterization of the GlyRα1 PPII helix. (A) Model of GlyRα1 with its predicted PPII helix in the TM3-4 loop (adapted from [44]. (B) Sequence of wildtype GlyR in comparison with group mutant at position 365–373. (C) SDS-PAGE of GlyRα1 as well as GlyRα1P1-5A mutant TM3-4 loops after over-expression in E. coli cells and purification via Ni-NTA column and gel filtration chromatography. Lane 1: α1 wildtype TM3-4 loop; lane 2: GlyRα1P1-5A TM3-4 loop; lane 3: protein standard. Position of TM3-4 loop and 12 kDa is indicated. (D) CD spectra of α1-wt (solid line) and GlyRα1P1-5A (dashed line). All spectra were measured in 10 mM K-phosphate, pH 7.4 in a 1 cm cuvette at 22 °C. Eight single spectra were summed and the reference spectrum (10 mM K-phosphate, pH 7.4) was subtracted.