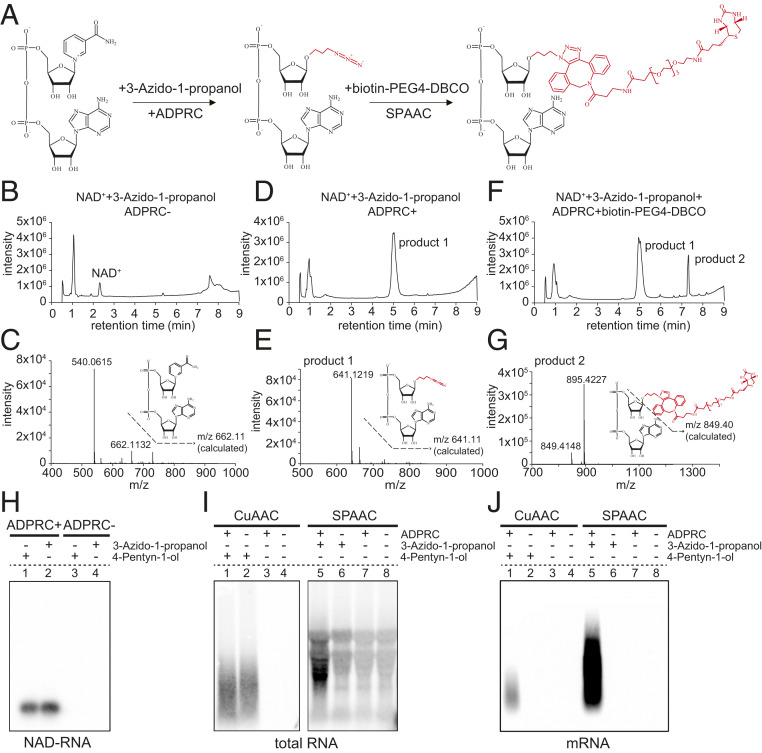
Fig. 1.
The establishment and advantage of the SPAAC-NAD reaction. (A) Schematic diagram of the SPAAC-NAD reaction. ADPRC catalyzes the reaction replacing the nicotinamide moiety of NAD+ with 3-azido-1-propanol, followed by SPAAC-based biotinylation with biotin-PEG4-DBCO. (B) HPLC chromatogram of the control reaction in which NAD+ was incubated with 3-azido-1-propanol in the absence of ADPRC. The NAD+ peak is marked. (C) Mass spectrum of the NAD+ peak in B. A compound of mass 662.113 matches NAD+ in mass. (D) HPLC chromatogram of the ADPRC-catalyzed reaction of NAD+ with 3-azido-1-propanol. A product is found compared to B. (E) Mass spectrum of the “product 1” peak in D. A compound of mass 641.121 matches the expected product as diagramed. (F) HPLC chromatogram of the ADPRC-catalyzed reaction of NAD+ with 3-azido-1-propanol followed by SPAAC with biotin-PEG4-DBCO. Product 2 is formed as compared to D. (G) Mass spectrum of the “product 2” peak in F. The compound of mass 849.414 matches the moiety in red in the molecular diagram of product 2. (H–J) Gel blot assays showing NAD-RNA capture. CuAAC- or SPAAC-NAD reactions were performed with an in vitro-transcribed NAD-RNA (H), 100 μg of total Arabidopsis RNAs (I), or 1 μg of Arabidopsis mRNAs (J). RNAs were incubated with/without 3-azido-1-propanol or 4-pentyn-1-ol in the presence or absence of ADPRC. The RNAs were resolved in 2% agarose gel and transferred to a nylon N+ membrane. Biotin-labeled products were probed with streptavidin–horseradish peroxidase, and signals were detected with a chemiluminescent nucleic acid detection module kit.