Significance
Both IL10 and ICOSL are risk alleles for inflammatory bowel disease (IBD). The role of IL-10 in preventing gut inflammation is well established experimentally and clinically. However, it is unclear whether and how ICOSL functions in the intestines, and how it might impact susceptibility to gut inflammation. We found that in mice, the absence of ICOSL is associated with increased accumulation of IL-10–producing CD4 T cells but dramatic reductions in anti-commensal antibodies, resulting in limited recognition of antigens implicated in the progression of IBD. Simultaneous disruption of both pathways, either genetically or transiently, resulted in colitis. Therefore, we have identified an IBD-relevant axis in intestinal immune regulation predicated on the cooperative functions of ICOSL and CD4 T cell–derived IL-10.
Keywords: IL-10, ICOSL, microbiota, anti-commensal antibodies
Abstract
Genome-wide association studies have identified ICOSLG, which encodes the inducible costimulator ligand (ICOSLG or ICOSL) as a susceptibility locus for inflammatory bowel disease. ICOSL has been implicated in the enhancement of pattern recognition receptor signaling in dendritic cells, induction of IL-10 production by CD4 T cells, and the generation of high-affinity antibodies to specific antigens—all of which can potentially explain its involvement in gastrointestinal inflammation. Here, we show that murine ICOSL deficiency results in significant enrichment of IL-10–producing CD4 T cells particularly in the proximal large intestine. Transient depletion of IL-10–producing cells from adult ICOSL-deficient mice induced severe colonic inflammation that was prevented when mice were first treated with metronidazole. ICOSL-deficient mice displayed reduced IgA and IgG antibodies in the colon mucus and impaired serum antibody recognition of microbial antigens, including flagellins derived from mucus-associated bacteria of the Lachnospiraceae family. Confirming the synergy between ICOSL and IL-10, ICOSL deficiency coupled with CD4-specific deletion of the Il10 gene resulted in juvenile onset colitis that was impeded when pups were fostered by ICOSL-sufficient dams. In this setting, we found that both maternally acquired and host-derived antibodies contribute to the life anti-commensal antibody repertoire that mediates this protection in early life. Collectively, our findings reveal a partnership between ICOSL-dependent anti-commensal antibodies and IL-10 in adaptive immune regulation of the microbiota in the large intestine. Furthermore, we identify ICOSL deficiency as an effective platform for exploring the functions of anti-commensal antibodies in host–microbiota mutualism.
The establishment and maintenance of immune homeostasis with the diverse community of microbes that inhabit the intestine is a dynamic process involving multiple cellular and molecular mediators. A major player in this process is the immunoregulatory cytokine interleukin-10 (IL-10), a dominant functional product of intestinal CD4+ T regulatory (Treg) cells at steady state. Germline deletion of Il10, targeted ablation in CD4 T cells (1), or just Foxp3+ cells (2), but not myeloid cells (3–6), can result in spontaneous adult-onset inflammation. IL10 has been identified by genome-wide association studies as a risk allele for both Crohn’s disease and ulcerative colitis, the two major types of inflammatory bowel disease (IBD). Moreover, humans with impaired expression of the receptor for IL-10 develop severe pediatric onset IBD (7). Thus, because of its critical role in gut immune regulation, elucidation of the molecular mechanisms that intersect the IL-10 pathway, and the circumstances surrounding those intersections, can identify avenues whereby host–microbiota mutualism is achieved and/or can potentially be reinforced to prevent gut inflammation.
The inducible T cell costimulator ligand (ICOSL) is a member of the B7 family of molecules that is constitutively expressed on antigen-presenting cells including B cells and dendritic cells (8–10). Via its interaction with the T cell–expressed ICOS receptor, ICOSL is able to modulate T cell activation and differentiation (11, 12). In humans, partial loss-of-function polymorphisms at chromosome 21q22, which encompasses ICOSLG (the gene encoding ICOSL), have also been associated with IBD as well as celiac disease—immune-mediated chronic inflammatory disorders of the gastrointestinal tract (13–15). One such IBD-associated polymorphism contributes to impaired dendritic cell production of proinflammatory cytokines in response to pathogen recognition receptor stimulation (16), demonstrating that impaired ICOSL function can adversely affect microbiota-induced innate immune responses.
ICOSL is also critical for the successful interaction of B and T cells in response to antigenic challenge. Specifically, during the germinal center reaction, B cell–expressed ICOSL interacts with ICOS on T follicular helper (Tfh) cells, promoting the retention of Tfh cells in the late germinal center reaction. In the absence of this interaction, developing Tfh cells lose their lineage-specific phenotype and leave the germinal center, eliminating T cell help in antibody production (17, 18). As evidence of the importance of this signal, complete deficiency of ICOSL or ICOS in humans results in combined immunodeficiency involving recurrent bacterial and viral infection and abnormal B cell maturation (19, 20).
Unlike in humans, ICOSL deficiency is largely inconsequential in laboratory mice maintained under specific pathogen-free conditions and thus “naïve” to the types of antigens encountered by humans. However, even in this state of restricted antigen exposure, antibody responses are still an essential component of the immune apparatus that enables peaceful coexistence with the microorganisms that inhabit barrier sites such as the intestines. These antibodies can be either broadly reactive, thymus (T)-independent immunoglobulins recognizing conserved bacterial moieties or high-affinity, antigen-specific antibodies, the generation of which requires T cell help (21–28). In experimental settings, the importance of T cells for antibody responses is usually based on whether such responses exist, or can be induced, in T cell-deficient mice (22, 26, 27, 29). Given the role of ICOSL in Tfh retention, the T-dependent anti-commensal antibody repertoire of ICOSL-deficient mice at steady state should resemble that of T cell–deficient mice. Moreover, ICOSL-deficient mice also retain much of the CD4 T cell compartment, including the various subsets of mucosal effector and regulatory T cells known to participate in intestinal immune tolerance (30–32). Arguably, this makes murine ICOSL deficiency a superior model with which to study the functional positioning of T-dependent anti-commensal antibodies within the larger immunoregulatory network of the gut.
Here we show that at homeostasis, and similar to ICOS-deficient mice (33), ICOSL-deficient mice harbor increased frequencies and numbers of IL-10–producing CD4 T cells particularly in the proximal colon lamina propria. Strikingly, transient depletion of IL-10–producing cells resulted in rapid onset of severe colonic inflammation that was also largely restricted to the proximal colon. Conversely, we observed significantly diminished colon-associated Tfh cells and IgA+ and IgG+ plasma cells as well as serum- and mucus-associated IgA and IgG in ICOSL-deficient mice, which suggest that the elevated IL-10 is induced to counterbalance these antibody deficits. In support of this, ICOSL-deficient mice displayed reduced serum antibody recognition of multiple bacterial antigens including flagellin antigens derived from several members of the family Lachnospiraceae, which are known to enrich within the colon mucus layer (34–36). Furthermore, CD4-specific ablation of Il10 coupled with germline deletion of Icosl predisposed to early onset intestinal inflammation that was delayed when the mutant pups was fostered by ICOSL-sufficient dams. Importantly, both maternally transmitted and host-derived ICOSL-dependent antibodies appear to be involved in limiting this early-life reactivity to the microbiota. Collectively, our data identify a synergy between two IBD-related pathways—T cell–derived IL-10 and ICOSL-dependent anti-commensal antibodies—that promotes mutualism with the gut microbiota.
Results
Transient Depletion of IL-10–Producing Cells from Adult ICOSL-Deficient Mice Results in Colonic Inflammation.
We previously reported that deficiency of ICOS, the T cell–expressed receptor for ICOSL, impairs the functional stability of Foxp3+ Treg cells (33). Surprisingly, we have found that ICOSL-deficient mice harbor wild-type (WT) levels of colonic Foxp3-expressing cells (SI Appendix, Fig. S1 A–C). Importantly, using our IL-10 reporter transgenic (10BiT) mice (37), we noticed that similar to ICOS-deficient mice, ICOSL-deficient mice displayed significant increases in colonic IL-10–producing cells and overall IL-10 protein output of total lamina propria CD4 T cells (SI Appendix, Fig. S1 A and D–F). Therefore, independent of any effect on Treg cells, ICOSL–ICOS pathway deficiency is associated with increased IL-10 in the colon. Importantly, the differences in IL-10–producing cells did not appear to be due to microbiota differences as analysis of the fecal microbiota of WT and Icosl−/− cohorts revealed no major compositional differences (SI Appendix, Fig. S2).
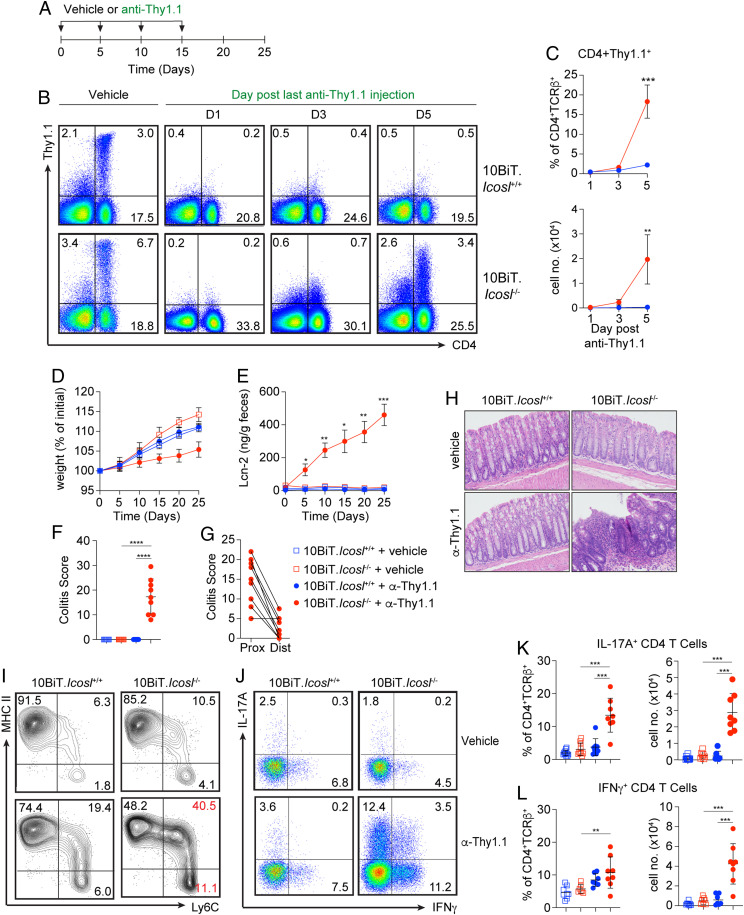
To begin to explore the significance of the increased IL-10 that emerges in the absence of ICOSL, we took advantage of a key feature of the 10BiT mice—surface expression of the Thy1.1 reporter molecule (37). This allows for transient depletion of IL-10–expressing cells from healthy adult mice, enabling strict temporal control of the IL-10 disruption. 10BiT.Icosl+/+ or 10BiT.Icosl−/− mice received intraperitoneal injections of anti-Thy1.1 or vehicle (phosphate-buffered saline [PBS]) every 5 d for 15 d. Body weight and fecal Lcn-2 were measured every 5 d, and mice were euthanized for analysis of colitis indices on day 25 (Fig. 1A). Anti-Thy1.1 treatment resulted in efficient depletion of IL-10–competent cells from the colonic lamina propria (Fig. 1 B and C). Interestingly, in 10BiT.Icosl−/− mice, the initial re-emergence of IL-10–competent cells could be seen by 5 d post antibody treatment, whereas 10BiT.Icosl+/+ mice remained fully depleted (Fig. 1 B and C), supporting the notion of a constant and increased demand for IL-10 production in the absence of ICOSL. Accordingly, anti-Thy1.1–treated 10BiT.Icosl−/− mice rapidly developed signs of inflammation including diminished weight gain and a rapid spike in fecal Lcn-2 even after a single injection of anti-Thy1.1 (Fig. 1 D and E). In contrast, nondepleted 10BiT.Icosl−/− and 10BiT.Icosl+/+ mice as well as depleted 10BiT.Icosl+/+ mice continued to gain weight and their fecal Lcn-2 levels remained low (Fig. 1 D and E). The development of colitis in depleted 10BiT.Icosl−/− mice was confirmed by histological evaluation on day 25 and revealed that colitis was predominantly confined to the proximal colon (Fig. 1 F–H).
Fig. 1.
Transient depletion of IL-10–producing cells from ICOSL-deficient mice results in colonic inflammation. (A) Experimental design for Thy1.1+ cell depletion experiments. (B) Representative flow cytometry plots demonstrating the impact of anti-Thy1.1 treatment on Thy1.1 expression on colon lamina propria cells of 10BiT.Icosl+/+ or 10BiT.Icosl−/− mice 1, 3, or 5 d after anti-Thy1.1 injection. (C) Graphs displaying frequencies (Upper) and numbers (Lower) of CD4+TCR-β+Thy1.1+ cells from mice analyzed as in B; n = 9 mice/group. (D) Percent change in weight and (E) fecal Lcn-2 levels measured every 5 d from start of anti-Thy1.1 injection. (F) Total histopathological scores of colonic tissue sections from all mice at day 25. (G) Histopathological scores of proximal (Prox) and distal (Dist) colon sections. (H) Representative hematoxylin and eosin–stained photomicrographs depicting the colonic inflammation observed in mice examined as in F and G. n = 8 to 10 mice/group. (I) Representative flow cytometry plots demonstrating colonic “monocyte waterfall” cell subsets from WT and Icosl−/− mice at 5 d after vehicle or anti-Thy1.1 injection. (J) Representative flow cytometry plots showing IFNγ and IL-17A expression by CD4 T cells in the colon lamina propria of mice analyzed in D–H. Scatter plots show frequencies (Left) and numbers (Right) of IL-17A+ (K) and IFNγ+ (L) CD4 T cells from mice analyzed as in A. n = 7 to 9 mice/group. Error bars represent mean ± SD; P values were calculated by ANOVA for repeated measures with Bonferroni correction (B–E) or by ANOVA followed by Tukey’s multiple comparisons test (F, G, K, L). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. All data are compiled from two (B, C, I, K, L) or three (E–G) independent experiments.
Previous studies have shown that ICOSL deficiency can impair the activation and proinflammatory potential of myeloid lineage cells (16, 38, 39), yet in our studies, mice devoid of ICOSL deficiency predisposed to a worse inflammatory outcome than did ICOSL sufficiency. Nevertheless, we sought to determine whether there was a myeloid cell defect that contributed to disease susceptibility in our model. Importantly, at steady state, and prior to the depletion of IL-10–producing cells, we did not detect any differences in the frequencies and numbers of lamina propria monocytes or macrophages in ICOSL-deficient mice relative to ICOSL-sufficient mice (SI Appendix, Fig. S3 A and B). Furthermore, using CD86 upregulation as an indicator of myeloid cell activation, we found similar levels of expression by monocytes, newly differentiated as well as mature macrophages, and dendritic cells in the lamina propria of the proximal and distal colon, suggesting that these cells were not hyper activated (SI Appendix, Fig. S3 C and D). However, within 5 d of anti-Thy1.1 treatment in vivo, and consistent with disruption of IL-10 signaling (5, 40), we observed an increase in monocyte recruitment and differentiation into inflammatory macrophages in the colonic lamina propria of ICOSL-deficient mice (Fig. 1H). By day 25, the colitis appeared to be sustained in a T cell–driven manner by robust frequencies of Th1 and Th17 lymphocytes in the colon lamina propria (Fig. 1 I–K). Collectively, these findings demonstrate that sustained expression of both IL-10 and ICOSL is essential for the maintenance of intestinal immune homeostasis, especially in the ascending colon.
Divergent Colon-Associated IL-10 and Tfh Cell Responses in the Absence of ICOSL.
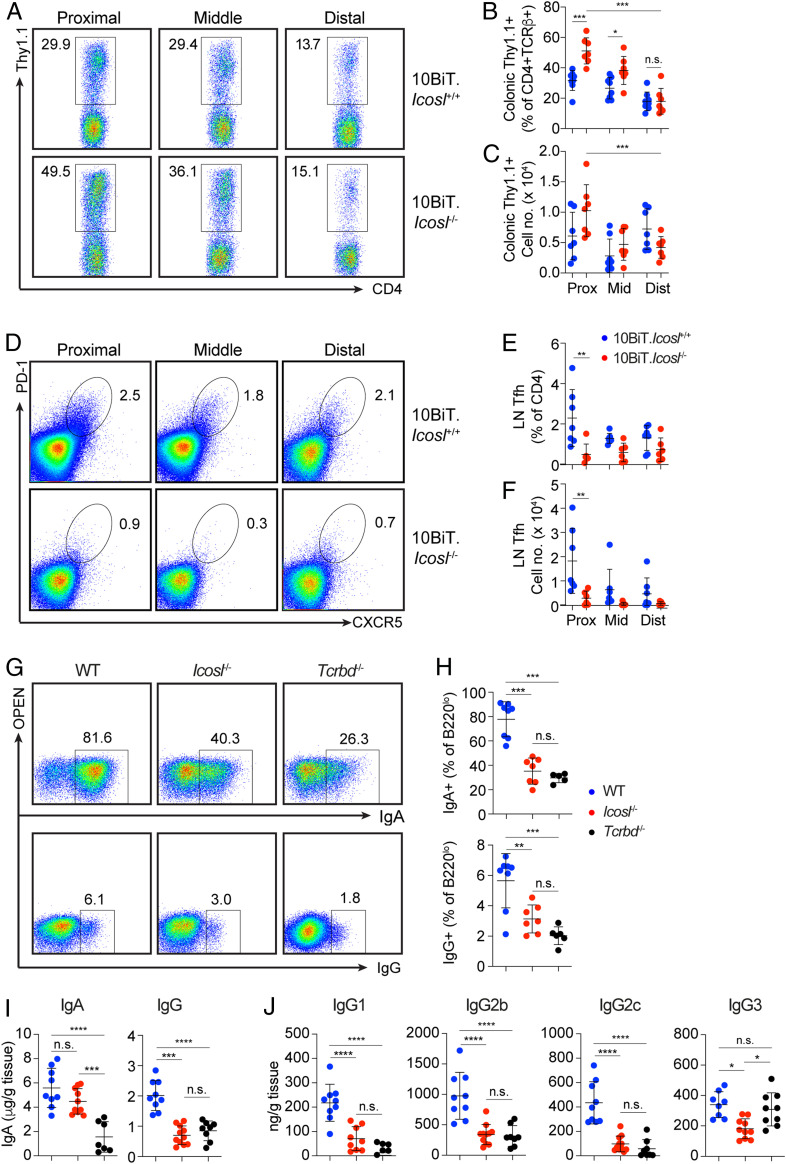
Based on the localization of inflammation to the proximal colon in Fig. 1, we next determined the impact of ICOSL deficiency on the expression of IL-10 by CD4 T cells along the length of the colon. We observed elevated frequencies of IL-10–producing CD4 T cells in the proximal relative to the distal colon in Icosl+/+ mice. Relative to the distal colon, the proximal colon of ICOSL-sufficient mice is enriched with IL-10–producing CD4 T cells (Fig. 2A, Top). However, the frequencies of these cells in the proximal colon are significantly increased in the absence of ICOSL (Fig. 2 A–C). In fact, on average, IL-10–competent cells accounted for ∼50% of all CD4+TCRβ+ cells in the proximal colon of Icosl−/− mice (Fig. 2 A–C).
Fig. 2.
T cell–derived IL-10 is elevated, whereas Tfh and anti-commensal antibodies are reduced in the colons of ICOSL-deficient mice. (A) Representative flow cytometry plots showing CD4+Thy1.1+ cells from the lamina propria of the proximal, middle, and distal colon of 10BiT.Icosl+/+ and 10BiT.Icosl−/− mice. Graphs show (B) frequencies and (C) numbers of cells per mouse analyzed as in A. (D) Representative flow cytometry plots showing Tfh cells in the lymph nodes draining the proximal, middle, and distal colon of 10BiT.Icosl+/+ and 10BiT.Icosl−/− mice. Graphs show (E) frequencies and (F) numbers of cells per mouse analyzed as in D. Plots in A and D are gated on CD4+TCRβ+ cells, and numbers represent the percentage of cells in the gate shown. 10BiT.Icosl+/+ n = 7, 10BiT.Icosl−/− n = 6. Data from one of two independent experiments are shown. (G) Representative flow cytometric analysis of IgA and IgG expression by B220lo cells from the colonic lamina propria of WT, Icosl−/−, or Tcrbd−/− mice. (H) Frequency of B220lo cells expressing IgA or IgG as depicted in A. WT, n = 8; Icosl−/−, n = 7; Tcrbd−/−, n = 6. Data are compiled from two independent experiments. (I) ELISA of total IgA and IgG in colonic mucus from WT, Icosl−/−, or Tcrbd−/− mice. WT, n = 9; Icosl−/−, n = 11; Tcrbd−/−, n = 8. (J) ELISA of IgG1, IgG2b, IgG2c, and IgG3 in colonic mucus from WT, Icosl−/−, or Tcrbd−/− mice. WT, n = 9; Icosl−/−, n = 10; Tcrbd−/−, n = 9. Data are compiled from three independent experiments. In all graphs, each symbol represents an individual mouse; error bars represent mean ± SD. P values were calculated by ANOVA followed by Tukey’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The interaction of ICOSL on B cells with ICOS on Tfh cells is essential for the retention of Tfh cells within the germinal center. In the absence of this interaction, developing Tfh cells lose their lineage specific phenotype and leave the germinal center, and thus antibody production is impaired (17, 18). In general, Tfh cells are rare in naïve specific pathogen-free mice but detectable in gut-associated lymphoid tissues in which they develop in a commensal-dependent manner (41). In WT mice, Tfh cells were most prevalent in the lymph nodes draining the proximal colon with reduced frequencies and numbers in the middle and distal regions (Fig. 2 D–F). In ICOSL-deficient mice, Tfh cells were reduced across all colon-draining nodes, with the most significant difference relative to WT mice detected in the proximal region, the same region where we observed the largest and increase in IL-10–producing CD4 T cells.
Reduced Colon-Associated Antibody Responses in ICOSL-Deficient Mice.
We next examined the impact of ICOSL on homeostatic levels of anti-commensal antibodies, by measuring the levels of colon-associated IgA and IgG, the two most common classes of switched antibodies induced by commensals both without and with the help of T cells. For comparison, we included TCRβ x TCRδ double-deficient (Tcrbd−/−) mice which are incapable of generating T-dependent antibodies. Like Tcrbd−/− mice, Icosl−/− mice displayed significantly reduced frequencies of IgA- and IgG-secreting cells in the colonic lamina propria (Fig. 2 G and H). At steady state, IgG is not readily detectable in murine feces (42); therefore, we focused our comparisons on the colon mucus aspirate. In the colon mucus, unlike the significant drop in IgA observed in Tcrbd−/− mice, the IgA levels detected in Icosl−/− mice were not substantially changed (Fig. 2I) and may represent a compensatory increase in T-independent IgA production amid reduced ICOSL-dependent IgA in Icosl−/− mice. However, both mutant strains displayed similar reductions in total IgG levels in the colon mucus (Fig. 2I). We also examined the relative levels of the four murine IgG subtypes in the serum and colon mucus of ICOSL-deficient mice and compared to T cell–deficient mice. We found statistically significant reductions in serum IgG1 and IgG2c but not IgG2b or IgG3 in both Icosl−/− and Tcrbd−/− mice (Fig. 2J). In the colon mucus, there were significant reductions in all IgG subtypes in both Icosl−/− and Tcrbd−/− with the exception of IgG3, which was unchanged in Tcrbd−/− mice (Fig. 2J). These results demonstrate the existence of similar colon-associated anti-microbial antibody alterations in ICOSL- and TCR-deficient mice and suggest similar deficits in antibody-mediated interactions with commensals.
ICOSL Deficiency Leads to Reduced Serum Antibody Recognition of Antigens Derived from Diverse Bacteria including Flagellated and Mucus-Associated Commensals.
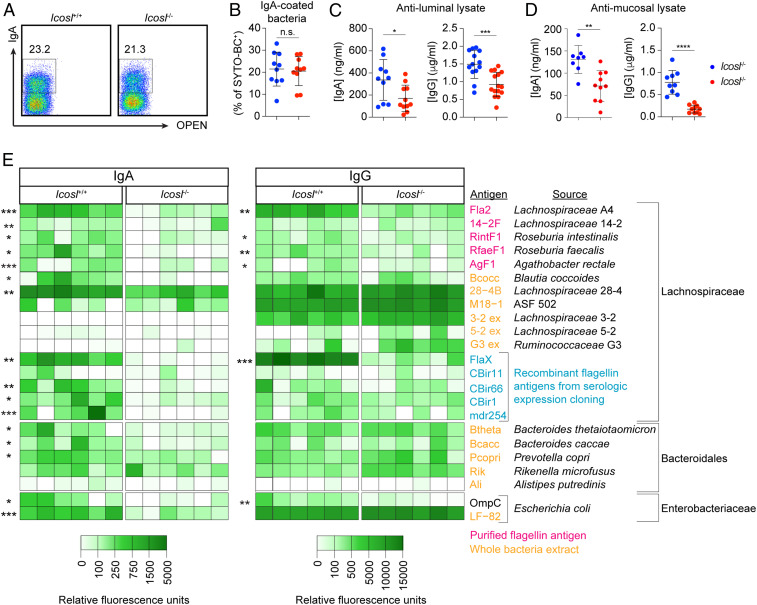
Anti-commensal IgA and IgG antibodies are known to be present in the circulation (27), and both Tcrbd−/− and Icosl−/− mice displayed reduced serum IgA and IgG relative to WT mice (SI Appendix, Fig. S4). To understand whether and how host–microbe interactions are disrupted in the absence of ICOSL, we examined the impact of ICOSL deficiency on the antibody-mediated recognition of whole commensal organisms as well as commensal antigens. Since a large fraction of fecal microbes is actively bound by predominantly T-independent IgA (25, 26), we first compared the relative frequencies of IgA-bound bacteria in the colon lumen of Icosl+/+ and Icosl−/− mice. ICOSL deficiency did not lead to reduced IgA coating of luminal bacteria (Fig. 3 B and C). This eliminated IgA-based sorting as a means to identify microbes that are specifically targeted by ICOSL-dependent antibodies. Moreover, we did not reliably detect IgG-bound microbes or binding of serum IgG to luminal bacteria, as has been previously described (21). Thus, the differences in antibody levels between WT and ICOSL-deficient mice did not appear to impact antibody coating of luminal microbes. We then harvested luminal and mucus-associated bacteria from Icosl+/+ and Icosl−/− mice and generated crude lysates that we then used in an enzyme-linked immunosorbent (ELISA)–based assay to “capture” antibodies present in paired serum. Using this system, we found significant reductions in the levels of anti-luminal lysate IgA and IgG, in Icosl−/− relative to Icosl+/+ mice (Fig. 3D), and an even more dramatic diminution in serum IgA and IgG binding to mucus lysates (Fig. 3E). Considering that luminal and mucus communities continually admix via bidirectional transfer (36), these data support the notion that mucus-associated communities in particular elicit robust ICOSL-dependent antibody responses.
Fig. 3.
Reduced antibody binding to antigens derived from predominantly mucus-associated bacteria in ICOSL-deficient mice. (A) Representative flow cytometric analysis of IgA-coating of bacteria (SYTO-BC+) from fecal pellets of Icosl+/+ or Icosl−/− mice. (B) Frequency of bacteria coated with IgA as analyzed in A. Data are compiled from three independent experiments. n = 10 mice/group. ELISA of matched serum IgA (Left) and IgG (Right) binding to crude bacterial lysates prepared from (C) luminal contents or (D) mucus from WT or Icosl−/− mice. Icosl+/+, n = 13; Icosl−/−, n = 15. In B–D, data are compiled from three independent experiments. Error bars represent mean ± SD, and P values were calculated by ANOVA test followed by Tukey’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001. (E) Heatmap showing relative fluorescence intensity of IgA (Left) and IgG (Right) binding to microbial antigens or lysates. Values were normalized to fluorescence, if any, detected when each antigen was probed with Rag1−/− mouse serum. Asterisks denote antigens or lysates for which there was a statistically significant difference in fluorescence intensity between Icosl−/− and Icosl−/− serum. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data are from one of two individual experiments.
Bacteria belonging to the Lachnospiraceae family are enriched among mucus-associated communities (34–36). Moreover, Crohn’s disease patients display seroreactivity to Lachnospiraceae flagellin antigens A4-Fla2 and FlaX, which, along with reactivity to CBir1 flagellin and Escherichia coli outer membrane protein C (OmpC), correlate with early postoperative recurrence of Crohn’s disease (43–45). Therefore, to determine the origin and types of antigens that drive ICOSL-dependent antibody responses, we performed a microbial antigen array (46) in which purified antigens or whole extracts of bacteria from an “in-house” library were screened for reactivity to serum IgA and IgG from Icosl+/+ and Icosl−/− mice. We tested a collection of purified flagellins or recombinant flagellins generated by serologic expression cloning as well as whole-cell extracts of several species of Lachnospiraceae, five members of the order Bacteroidales, and E. coli. There was reduced serum IgA from Icosl−/− mice reactive to almost all Lachnospiraceae flagellin antigens tested as well as to extracts of Bacteroides thetaiotaomicron, Bacteroides caccae, and Prevotella copri (Fig. 3E, Left). In addition, IgG reactivity to several Lachnospiraceae flagellin antigens, including most notably A4Fla2 as well as FlaX, was also significantly reduced in ICOSL-deficient mice (Fig. 3E, Right). Collectively, our results demonstrate that in the absence of ICOSL-dependent anti-commensal antibodies, there is reduced IgA and IgG targeting antigens derived from multiple species of anaerobic bacteria, including antigens previously associated with active IBD. Consistent with this, treatment with metronidazole, which depletes anaerobes, completely inhibited development of colitis when ICOSL-deficient mice were depleted of IL-10–producing cells (SI Appendix, Fig. S5). Disease was also partially suppressed by vancomycin but unimpacted by colistin, which broadly target Gram+ and Gram− bacteria, respectively. Thus, the colitis that develops when IL-10–producing cells are transiently removed from mice with a deficiency of ICOSL-dependent anti-commensal antibodies appears to be driven by predominantly Gram+ anaerobic bacteria.
Coablation of Icosl and CD4 T Cell–Derived Il10 Predisposes to Early-Onset Colitis.
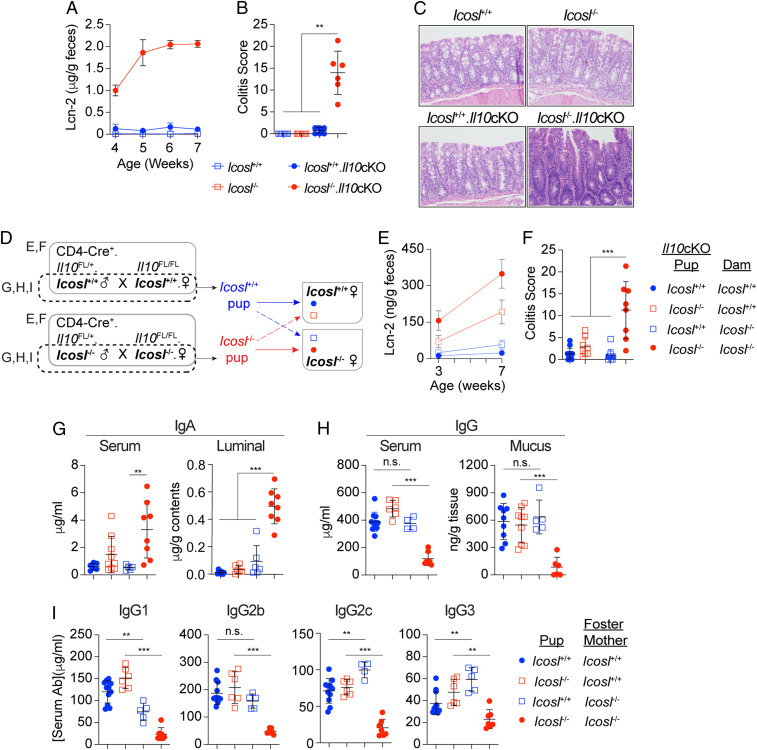
All of the foregoing results suggest a cooperative relationship between IL-10 and ICOSL-dependent anti-commensal antibodies in the regulation of colonic immune homeostasis at least in adulthood. To determine whether CD4 T cells are the primary source of this synergistic IL-10 and to examine the possible necessity of this axis for gut immune homeostasis in early life, we eliminated CD4 T cell expression of IL-10 in ICOSL-deficient mice. We crossed mice in which IL-10 deficiency is restricted to CD4–expressing cells (Il10 conditional knockout, Il10cKO) (1) with Icosl−/− mice to generate mice deficient for only ICOSL (Icosl−/−), only T cell–derived IL-10 (Il10cKO), both (Icosl−/−.Il10cKO), or neither (Icosl+/+). Kinetic analysis of fecal lipocalin-2 (Lcn-2) showed elevated expression as early as the fourth week of life in Icosl−/−.Il10cKO mice but not in Icosl+/+, Icosl−/−, or Il10cKO animals (Fig. 4A). The fecal Lcn-2 levels increased rapidly over the next few weeks, reaching a plateau by the sixth week of life. Histological analysis at 7 wk of age confirmed the development of severe colitis in Icosl−/−.Il10cKO mice while the other groups remained disease-free during the period of analysis (Fig. 4 B and C). CD4-specific IL-10 deficiency in mice has long been known to predispose to adult-onset colitis (1), and as expected, Il10cKO mice eventually experienced dramatic disease-related weight loss starting at around 18 wk of age, and all were severely colitic when euthanized at 27 to 28 wk of age (SI Appendix, Fig. S6). This argues that the absence of ICOSL can measurably hasten colitis onset in the absence of T cell–derived IL-10 and demonstrates that CD4 T cells are the primary source of the IL-10 that counters ICOSL deficiency.
Fig. 4.
Maternal and host-derived ICOSL-dependent antibodies in combination with IL-10 help to promote intestinal immune homeostasis in early life. (A) Longitudinal Lcn-2 levels detected in feces of Icosl+/+, Icosl−/−, Icosl+/+.Il10cKO, and Icosl−/−.Il10cKO mice from 4 to 7 wk of age. (B) Histopathological scoring of hematoxylin and eosin–stained sections and (C) representative photomicrographs from mice of all four genotypes analyzed at 8 wk of age; n = 8 mice/group (20× magnification). (D) Experimental design for cross-fostering experiments featuring Icosl+/+ and Icosl−/− mice with (solid line) or without (broken line) mutations associated with condition deletion of Il10. Graphs summarizing (E) Fecal Lcn-2 at 3 and 7 wk and (F) histopathological scores at week 7; n = 8 to 13 mice/group. Scatter plots show levels of serum and luminal IgA (G), serum and mucus IgG (H), and serum IgG1, IgG2b, IgG2c, and IgG3 (I) for 3-wk-old Icosl+/+ and Icosl−/− pups housed as described in F. n = 5 to 11 mice/group. For all graphs, error bars represent mean ± SD. P values were calculated by ANOVA followed by Tukey’s multiple comparisons test; *P < 0.05, **P < 0.01, ***P < 0.001. Data are representative of two (A–C) or four (D–I) experiments.
Early-Onset Colitis that Develops in Icosl−/−.Il10cKO Mice Is Delayed in Mice Fostered by ICOSL-Sufficient Dams.
Anti-commensal antibodies transferred from mother to offspring via the placenta and/or the breast milk can help to tolerize the newborn to the encroaching microbiota. In theory, maternally derived antibodies can be either T dependent or T independent, but it has been reported that the major players in early-life homeostasis are T-independent IgA, IgG2b, and IgG3 (22). We thus sought to determine whether an ICOSL-sufficient nursing mom could help rescue the early-onset disease that develops in Icosl−/−.Il10cKO mice, which would provide further proof of the importance of ICOSL-dependent antibodies in mitigating this inflammatory reaction. We conducted a series of timed matings utilizing Icosl+/+ or Icosl−/− mice that importantly were negative for Cre recombinase (Fig. 4D). In the first instance, the dams were also homozygous for LoxP-flanked Il10 alleles (Il10FL/FL.Icosl+/+ or Il10FL/FL.Icosl−/−) (1). These mice were mated with Il10FL/+ males that were positive for Cre recombinase under the control of the Cd4 promoter. This allowed for the introduction of the mutations enabling conditional deletion of Il10 (Il10cKO) in the progeny born to both WT and ICOSL-deficient dams. Within 12 h after birth, half of the male and female progeny in each litter was removed and fostered by the dam of the opposing Icosl genotype. Consistent with our earlier observation, Icosl+/+.Il10cKO mice that remained with their Icosl+/+ birth mother remained disease free throughout the observation period (Fig. 4 E and F). Icosl−/−.Il10cKO pups fostered by Icosl+/+ dams displayed low but detectable levels of fecal Lcn-2 at weaning, but the levels increased gradually thereafter. At 7 wk of age, these mice were found to have limited disease which suggests that maternally acquired ICOSL-dependent factors help to limit early-life immune reactivity to the microbiota in the large intestine.
Early-Life Intestinal Immune Homeostasis Involves ICOSL-Dependent Antibodies that Are both Maternally Acquired and Host Derived.
The discordance between fostered mice was more dramatic in mice housed with Icosl−/− dams. Here, as expected, Icosl−/−.Il10cKO mice showed elevated Lcn-2 as early as 3 wk which increased further after weaning, and the majority of mice were severely colitic by 7 wk of age (Fig. 4 E and F). In contrast, their Icosl+/+.Il10cKO cage mates were mostly free of early signs of inflammation, suggesting a possible contribution of host-derived antibodies as well in limiting microbiota reactivity. Therefore, to determine the relative contributions of maternal and host-derived ICOSL-dependent IgA and IgG antibodies to early-life homeostasis, we performed additional cross-fostering experiments but without the additional variable of disease susceptibility as a result of conditional deletion of Il10. Icosl+/+ and Icosl−/− littermate females were weaned together and cohoused until they reach sexual maturity. These females were then separated and subjected to timed mating with proven males of the same genotype. Pups were swapped within the first 12 h of life, and antibody levels were determined at 21 d of age. There was no significant difference in the levels of serum or colon luminal IgA among Icosl+/+ and Icosl−/− mice raised by Icosl+/+ dams (Fig. 4G). In contrast, we observed significant increases in both serum and luminal IgA in Icosl−/− pups, relative to their Icosl+/+ foster siblings all raised by Icosl−/− dams. This suggests that in the absence of maternal ICOSL-dependent anti-commensal IgA, host production of ICOSL-independent IgA in early life is increased. These results are consistent with our earlier observation of near-WT levels of colon mucus IgA in ICOSL-deficient adult mice (Fig. 2I).
Interestingly, Icosl−/− pups raised by their Icosl−/− mothers had significantly reduced levels of total IgG in both the serum and colon mucus (Fig. 4H). This demonstrates that, in contrast to the elevated IgA, the IgG antibodies present at this stage of life are predominantly ICOSL dependent. As with IgA, the amount of total IgG in the serum and colon mucus of pups raised by Icosl+/+ dams was similar in Icosl+/+ and Icosl−/− pups, suggesting maternal origin. However, these IgG levels were matched by those of Icosl+/+ pups placed with Icosl−/− dams arguing that these antibodies can also originate in the pups and are likely increased to compensate for the absence of the maternal source. Analysis of the serum levels of individual subtypes of IgG showed a significant reduction in IgG1 in cross-fostered Icosl+/+ pups relative to their siblings that were left with their birth mothers (Fig. 4I, Left), indicating an important contribution from the mother to the early-life IgG1 pool. In contrast, the cross-fostered Icosl+/+ pups displayed significant increases in IgG2c and IgG3 relative to those left with their birth mothers (Fig. 4I) suggesting these subtypes are largely pup derived and likely amplified in the absence of maternal ICOSL-dependent antibodies. Altogether, these results demonstrate that that both maternal and host-derived IgG antibodies contribute to the early-life anti-commensal antibody pool and that the sustained levels of most subtypes are dynamically impacted by the relative contributions from each source. Moreover, they provide evidence that ICOSL-dependent anti-commensal antibodies are part of a mechanism capable of at least temporarily maintaining intestinal immune homeostasis in mice even in the absence of T cell–derived IL-10. Coupled with results presented in Fig. 1, these data further imply that the synergy between ICOSL-dependent antibodies and IL-10 is essential in early life and continues to be necessary for the long-term maintenance of host–microbiota mutualism.
Discussion
In this study, we have presented murine ICOSL deficiency as a T cell–replete model of anti-commensal antibody deficiency, displaying diminished recognition by IgA and IgG antibodies of antigens derived from predominantly mucus-associated bacteria. By studying the CD4 T cell compartment in these mice, we identified a heretofore unknown synergy between ICOSL-dependent anti-commensal antibodies and IL-10 in regulating proinflammatory responses to the colonic microbiota starting in early life and extending into adulthood. Our study demonstrates how ICOSL deficiency can facilitate comprehensive mechanistic exploration of the relationship between anti-commensal antibodies and other immune pathways in promoting and maintaining host–microbiota mutualism. We posit that transient disruption of IL-10 function coupled with ICOSL deficiency represents an inducible and highly reproducible model of microbiota-dependent colonic inflammation in otherwise immune-sufficient mice.
We and others have observed defective Treg cell survival and/or epigenetic stability in the absence of ICOS (33, 47–50), the T cell–expressed receptor for which ICOSL is presumed to be the sole ligand. Surprisingly, we found no Foxp3+ Treg cell deficits in ICOSL-deficient mice. Further work will be necessary to determine whether the ICOS receptor is indeed triggered by an alternative mechanism in the absence of ICOSL. Nevertheless, this unexpected result forced us to explore an alternative basis for the enhanced accumulation of IL-10–producing CD4 T cells observed in the colon of both ICOS-deficient (33) and ICOSL-deficient mice. Fortunately, however, it also enabled utilization of Icosl−/− mice without any confounding deficiencies in the Treg cell compartment. We also confirmed the absence of any effect of ICOSL deficiency on the differentiation and activation status of gut myeloid cells at steady state. These results strengthen our argument that the observed antibody deficiencies and elevated IL-10–producing CD4 T cells we detected at steady state are likely not due to defects in Treg or macrophage function. Instead, these phenomena appear to be inextricably linked, such that their combined disruption at any point in time predisposes to rapid-onset colonic inflammation.
Owing to the importance of ICOSL–ICOS in Tfh responses, ICOSL-deficient mice are a bona fide model of T-dependent anti-commensal antibody deficiency, featuring obvious advantages over the commonly utilized T cell–deficient mice. Most notably, the major compensatory change seemingly triggered by the Tfh deficiency in ICOSL-deficient mice is increased IL-10–producing CD4 T cells in the proximal colon. Obviously, this axis would have gone undetected if T cell–deficient mice were used instead, and thus our study highlights the value of lymphocyte-replete model systems for exploring the functions of anti-commensal antibodies. In general, ICOSL-deficient mice harbored antibody levels comparable to those of Tcrbd−/− mice, although there are noteworthy differences. For example, despite significant reductions in colonic antibody-secreting cells relative to WT mice, Icosl−/− mice harbored increased plasma cells relative to Tcrbd−/− mice. This led to significant differences in serum and mucus IgA between Icosl−/− and Tcrbd−/− mice. In fact, in the colon mucus, there was actually no statistically significant difference in IgA between WT and Icosl−/− mice. Furthermore, in 3-wk-old Icosl−/− pups raised by their Icosl−/− birth mothers, there was a significant increase in luminal IgA relative to the littermates that were fostered by Icosl+/+ dams. This is likely due to a compensatory increase and/or accumulation of mostly T-independent IgA when all sources of ICOSL-dependent antibodies are disrupted. Importantly, despite the near-normal levels of colon mucus IgA, Icosl−/− mice were still prone to colitis development when the IL-10–producing cells were depleted, and Icosl−/−.Il10cKO mice still developed early-onset inflammation. Therefore, regardless of the nature and level of the extra IgA generated in the absence of ICOSL, it is inadequate to control the organisms that drive the disease observed in both scenarios.
Both humans and mice express four different subclasses of IgG and it is generally accepted that the majority of T-dependent IgG antibodies, including those elicited by murine pathogens, are of the IgG1 subclass (42). In contrast, microbiota-reactive IgG2b and IgG3 antibodies are generated by predominantly TI mechanisms (22). Overall, we observed similar trends in the amounts of serum IgG antibodies in both Icosl−/− and Tcrbd−/− mice relative to WT mice. This included consistent reductions in IgG1 and IgG2c, but not IgG2b or IgG3, in the serum of both mutant strains. In the colon mucus, there were notable deviations from these trends in that the levels of IgG2b were reduced in both Tcrbd−/− and Icosl−/− mice. Furthermore, the levels of IgG3 were also reduced in Icosl−/− colon mucus as well as in the serum of 3-wk-old Icosl−/− pups raised by their isogenic mothers. The reasons for these discrepancies remain unclear.
The microbial density of the mucus layer is greater in the proximal relative to the distal colon (51). Our findings that Tfh cell responses are more robust in the lymph nodes draining this region, and that IL-10–producing CD4 T cells enrich in this region especially when Tfh are impaired, highlight the necessity of robust immune regulation in this locale to actively limiting deleterious responses to mucus-associated bacteria. Owing to their proximity to the epithelium, mucus-associated commensals would arguably be more likely to require T-dependent antibody-mediated control and more prone to trigger an inflammatory response if not adequately restrained. Acute antigenic challenge results in the expression of IL-10 by CD4+ T follicular regulatory (TFR) cells which in turn promotes the differentiation of plasma cells (52). In light of our findings, it seems plausible that the role of T cell–derived IL-10 in synergy with antibody responses to commensal antigens involves both promoting the generation of a subset of these antibodies and actively compensating for any shortfall in their production, all in an effort to limit immune reactivity to mucus-associated microorganisms.
A previous study demonstrated an essential role for IL-10 in limiting inflammatory responses in recently colonized mice (53). By examining ICOSL-deficient mice incapable of IL-10 production in CD4 T cells, we now show that this IL-10 is predominantly T cell derived. In addition, it was previously shown that peaceful intestinal colonization of neonatal mice requires maternally derived T-independent IgA and IgG antibodies that are acquired via the breast milk and efficiently bind to luminal microbes (22). Our study does not dispute these findings but identifies additional roles in this process for maternally acquired and host-derived ICOSL-dependent antibodies acting in concert with T cell–derived IL-10. Ablation of IL-10 expression either in the germline or solely in CD4 T cells has long been known to predispose to colitis (1, 54–56) contingent on the presence in the microbiota of specific pathobiont species (57–59). Importantly, we observed the expected delay in disease onset in mice deficient in only T cell–derived IL-10. However, in the absence of ICOSL, signs of disease were evident as early as weaning, suggesting that the presence of a robust repertoire of ICOSL-dependent anti-commensal antibodies can temporarily suppress the colitogenicity of even a “permissive” microbiota. These findings further suggest that the failure to progress to colitis, as sometimes occurs in this model, may be evidence of sustained ICOSL-dependent antibody-mediated control of mucus-associated communities.
Various microbes or microbial consortia can directly enhance IL-10–producing CD4 T cell accumulation in the colon or require IL-10 for their mutualistic existence in the intestine. These include Bacteroides fragilis, Bifidobacterium breve, a mixture of 17 microbes isolated from human feces and containing several species of Lachnospiraceae, and the commonly used eight-member consortium referred to as the altered Schaedler’s flora (53, 60–62). Our analysis identified microbial antigen extracts from Lachnospiraceae, Bacteroidales, and Enterobacteriaceae that were differentially recognized by antibodies from WT mice relative to ICOSL-deficient littermates. Elevated T cell production of IL-10 in the absence of these antibodies implies that impaired antibody control of such microbial antigens can give way to antigen-dependent expansion of IL-10–producing colonic CD4 T cells. It also suggests that induction of ICOSL-dependent antibodies and T cell–derived IL-10 may be simultaneous host adaptations to microbial occupation of a niche near the epithelium. Future exploration of the specific microbes that drive these responses could potentially identify novel antigen-specific approaches to bolster mucosal immune defenses.
Finally, our study demonstrates that ablation of ICOSL expression impacts the mucosal levels of both IgA and IgG. Moreover, we detected deficiencies in the binding of IgA and IgG antibodies to flagellin and other antigens when antibody production was impaired. Based on the quantities detected in WT mice and the dramatic reductions in Icosl−/− mice, we would predict that ICOSL-dependent antibodies of both isotypes are important for ensuring gut homeostasis, but additional studies will be needed to determine the potential synergy and/or division of labor between these two isotypes and even between the various subtypes of IgG. Our results showing reduced anti-flagellin IgA in Icosl−/− mice, coupled with a recent study demonstrating a protective role for anti-flagellin IgA in preventing the development of murine colitis in response to disrupted IL-10 signaling (63), supports a beneficial role for IgA in preventing the intestinal inflammation observed in this study. Importantly, our data also argue in favor of IgG recognition of microbial antigens, including robust responses to flagellin antigens associated with IBD patients (44, 45). Unlike IgA, anti-flagellin IgG was shown to enhance the severity of disease in mice subsequently administered dextran sulfate sodium (64), supporting the long-held notion that IgG, acting via Fcγ receptors, is involved in the pathogenesis and/or perpetuation of IBD (15, 44, 65–67). However, a protective role for anti-commensal IgG was suggested by our analysis of Icosl+/+ and Icosl−/− weanlings that were raised either by their birth mother or by a dam of the opposite genotype. In these experiments, ICOSL-dependent IgG antibodies, derived from both mother and pup, were revealed to be major contributors to the homeostatic IgG repertoire at weaning and likely figure prominently in limiting adverse events during the weaning reaction. Therefore, our study, in combination with recent studies demonstrating important functions of anti-commensal IgG antibodies under homeostatic conditions (21, 22), stresses the need for future reconciliation of these seemingly opposing roles of IgG in intestinal health and disease.
Materials and Methods
Mice.
All mice used in this study were on the C57BL/6 genetic background. Icos−/−, Icosl−/−, Tcrb−/−Tcrd−/− (Tcrbd−/−), Rag1−/−, and CD4Cre mice were originally purchased from Jackson Laboratory. Il10FLOX mice were generously provided by Dr. A. Roers. 10BiT mice have been previously reported (37). Unless otherwise stated, 10BiT.Icosl+/+ and 10BiT.Icosl−/− mice for individual experiments were either littermates or age- and sex-matched mice cohoused starting at weaning (DOL 21) until experiments were initiated at 8 wk of age. Tcrbd+/+ (WT) and Tcrbd−/− mice were littermates generated by mating Tcrbd+/− males and females. In comparisons of wild-type, Icosl−/−, and Tcrbd−/− mice, wild-type mice are littermates/cage mates pooled from both cohorts. Mice were bred and maintained under specific pathogen-free conditions at the University of Alabama at Birmingham in accordance with Institutional Animal Care and Use Committee guidelines.
Collection of Colon Luminal Contents and Mucus.
Fecal pellets were collected, and large intestines were removed and opened longitudinally. Solid (luminal) content removed by forceps. Tissue was rinsed with PBS before collection of mucus by gentle suction, as described. Importantly, the suction did not appear interfere with the plasma cells present in the underlying lamina propria (SI Appendix, Fig. S7). Mucus was resuspended in 200 μL PBS. Fecal pellets were suspended in 0.1 mg/mL PBS. All samples were homogenized and centrifuged briefly (2,000 × g) to remove debris. Supernatants were collected and centrifuged (11,000 × g) to pellet bacteria. This supernatant was collected for quantification of free antibody, whereas the bacterial pellet was resuspended in 200 μL B-PER II Bacterial Protein Extraction Reagent (Thermo Fisher Scientific) to prepare microbial lysate. All samples were frozen at −70 °C before use.
Mucosal Lymphocyte Isolation.
Large intestine was removed, stripped of mesenteric fat, flushed with sterile PBS, and opened longitudinally. Tissue was cut into 1 cm sections and incubated with rotation at 37 °C in 154 µg/L l-dithioerythritol and 2 µM EDTA in HBSS to remove epithelium. Remaining tissue was then incubated with rotation at 37 °C with 20 µg/mL DNase-I and 100 U/mL collagenase IV (Sigma-Aldrich), and tissue disruption was completed using a GentleMACS Dissociator (Miltenyi Biotec). Total lamina propria cells were purified on a 40%/75% percoll gradient by room temperature centrifugation for 20 min at 2000 rpm with no brake. Lymph nodes draining the proximal, middle, and distal colon, identified as previously described (68), were mechanically dissociated in RPMI. All cell suspensions were then filtered through 70 µM mesh strainer prior to counting and further analysis.
Antibodies and Flow Cytometry.
Cells were washed with PBS, blocked with 2.4G2 (BioXCell, 10 µg/mL), and then stained with LIVE/DEAD Fixable Near-IR (Invitrogen) and anti-mouse antibodies purchased from BioLegend: CD4 (GK1.5), TCRβ chain (H57-597), IL-17A (TC11-18H10.1), CD90.1 (OX-7), GL7 Antigen (GL7), and IgG (goat polyclonal); eBioscience: Foxp3 (FJK-16S), IFNγ (XMG1.2), and CXCR5 (SPRCL5); Invitrogen: PD-1 (J43), CD45R/B220 (RA3-682), and IgD (11–26); BD Pharmingen: CD95 (Jo2); or Southern Biotech: IgA (goat polyclonal). Cells were permeabilized for intracellular stains using the Foxp3/Transcription Factor Fixation/Permeabilization Kit. Cells for effector cytokine staining were stimulated at 37° in RPMI with 10% fetal bovine serum (FBS) (R10) with ionomycin (750 ng/mL), phorbol 12-myristate 13-acetate (50 ng/mL), and GolgiPlug (BD Biosciences) prior to blocking. All cells were fixed in 2% paraformaldehyde and acquired with an LSR II cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar).
ELISA of Free or Microbiota-Binding Antibody.
Plates were coated overnight at 4 °C in 100 uL of 10 µg/mL goat anti-mouse Ig (Southern Biotech) or microbial lysate diluted to 10 ug protein/mL as determined by Pierce bicinchoninic acid (BCA) Protein Assay Kit (Thermo Fisher Scientific). Plates were blocked 2 h with 1% bovine serum albumin (BSA) in PBS before addition of free antibody supernatants or, to lysate coated plates, mouse-matched serum. After 2 h incubation, Goat Anti-Mouse horseradish peroxidase-conjugated antibodies from Southern Biotech were used to detect IgA (1040-05), IgG (1031-05), IgG1 (1070-05), IgG2b (1090-05), IgG2c (1079-05), or IgG3 (1100-05). All assays were normalized to negative controls from Rag1−/− mice.
IL-10 ELISA.
CD4 T cells were magnetically sorted (Dynabeads Mouse CD4, Invitrogen) from colonic lamina propria cells and plated at 1 × 106/mL in R10 and anti-CD28 (37.51, BD Pharmingen) in flat-bottom 96-well plates coated with anti-CD3 (145-2C11) for 24 h. Supernatants were assayed with Mouse DuoSet IL-10 ELISA kit (R&D) according to the manufacturer’s instructions.
IL-10-Competent Cell Depletion and Analysis of Ensuing Disease.
Eight-week-old 10BiT.Icosl+/+ or 10BiT.Icosl−/− mice were injected intraperitoneally with 100 μL of 1 mg/mL anti-Thy1.1 (19E12, BioXCell) or vehicle (PBS) every 5 d for 10 or 15 d. Weights and fecal pellets were collected every 5 d. Fecal pellets were suspended at 0.1 mg/mL in 0.1% Tween 20 in PBS and fecal Lcn-2 was measured by Mouse DuoSet Lipocalin-2/NGAL ELISA kit (R&D Systems). In all experiments, mice were euthanized as indicated in the text on either day 15 or day 25. Representative sections of large intestine were fixed in 2% formalin and embedded in paraffin and 5 μm sections were cut and stained with hematoxylin and eosin . Histological scoring was performed in a blinded fashion by a board-certified pathologist.
Microbial Flow Cytometry.
Fecal pellets were collected into 500 μL of protease inhibitor mixture (Roche) before homogenizing and brief centrifugation (2,000 × g) to remove debris. Supernatant was centrifuged at 4,000 × g for 10 min, and the resulting bacterial pellet was washed in sterile bacterial staining buffer (1% BSA in PBS). Bacteria were stained on ice with anti-mouse IgA, washed, and resuspended in SYTO BC (Invitrogen) to stain for nucleic acids. SYTO BC gates for bacteria were determined by comparison to germ-free control pellets. Samples were acquired with an LSR II cytometer (BD Biosciences), and data were analyzed using FlowJo software (TreeStar).
Microbial Antigen Array.
Bacterial proteins and extracts were arrayed in triplicate on FAST 16 pad nitrocellulose slides (Maine Mfg.) at a concentration of 0.2 mg/mL using a Spotbot Personal Microarrayer (Korteks). Proteins were diluted from recombinant stocks to 10 mM Tris (pH 7.4), 20% glycerol, and 0.1% sodium dodecyl sulfate (SDS). Extracts were obtained by freeze-thawing whole cells in 10 mM Tris (pH 7.4) and 0.1% SDS three times then removing insoluble cell debris by centrifugation. Extracts were then brought to 20% glycerol before arraying. To probe the microarray, pads were blocked for 1 h in SuperBlock (Thermo Fisher) and then probed with mouse sera diluted 1 to 10 in SuperBlock for 1 h. Serum obtained from Rag1−/− mice was used as a negative control. The pads were washed three times in PBS w/0.05% Tween 20 and a mixture of goat anti-mouse IgA-Alexa 555 (Southern Biotech, 1040-32) and goat anti-mouse IgG (H+L)-Dylight 650 (Thermo Fisher Scientific, 84545) were applied at 1 to 1,000 (∼0.5 μg/mL) for 1 h in SuperBlock. The pads were then washed three times with PBS-Tween, air-dried, and scanned using a GenePix 4000B imager (Axion).
Statistical Analysis.
Statistical significance was calculated by unpaired Student’s t test or ANOVA as appropriate, using Prism software (GraphPad). All P ≤ 0.05 are considered significant and are referred to as such in the text.
Supplementary Material
Acknowledgments
We thank members of the Maynard laboratory for critical comments throughout this study and Drs. L. Harrington, F. Lund, and J. Kearney for helpful discussions and/or assistance with critical reagents. Funding for this study was provided by the NIH (Grant DK118386 to C.L.M.), Crohn’s and Colitis Foundation (Research Initiatives Award 558420 to C.L.M.), and the University of Alabama at Birmingham (UAB) Microbiome Center (C.L.M.). A.E.L. was supported by T32 AI007051 and K.M.K. by Institutional Research and Academic Career Development Award K12 GM088010. We acknowledge the following for their support of the UAB Microbiome Resource: Comprehensive Cancer Center (P30 AR050948), Center for Clinical Translational Science (UL1 TR001417), University Wide Institutional Core, Heflin Center for Genomic Sciences, and the UAB Microbiome Center.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2018278118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Roers A., et al., T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J. Exp. Med. 200, 1289–1297 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubtsov Y. P., et al., Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28, 546–558 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Siewe L., et al., Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur. J. Immunol. 36, 3248–3255 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Pils M. C., et al., Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. Eur. J. Immunol. 40, 443–448 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Zigmond E., et al., Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 40, 720–733 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Krause P., et al., IL-10-producing intestinal macrophages prevent excessive antibacterial innate immunity by limiting IL-23 synthesis. Nat. Commun. 6, 7055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glocker E. O., et al., Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361, 2033–2045 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling V., et al., Cutting edge: Identification of GL50, a novel B7-like protein that functionally binds to ICOS receptor. J. Immunol. 164, 1653–1657 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Wang S., et al., Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood 96, 2808–2813 (2000). [PubMed] [Google Scholar]
- 10.Yoshinaga S. K., et al., Characterization of a new human B7-related protein: B7RP-1 is the ligand to the co-stimulatory protein ICOS. Int. Immunol. 12, 1439–1447 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Yoshinaga S. K., et al., T-cell co-stimulation through B7RP-1 and ICOS. Nature 402, 827–832 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Dong C., et al., ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 409, 97–101 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Dubois P. C., et al., Multiple common variants for celiac disease influencing immune gene expression. Nat. Genet. 42, 295–302 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke A., et al., Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 42, 1118–1125 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jostins L.et al.; International IBD Genetics Consortium (IIBDGC) , Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedl M., Lahiri A., Ning K., Cho J. H., Abraham C., Pattern recognition receptor signaling in human dendritic cells is enhanced by ICOS ligand and modulated by the Crohn’s disease ICOSLG risk allele. Immunity 40, 734–746 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J. Y., et al., The transcription factor KLF2 restrains CD4+ T follicular helper cell differentiation. Immunity 42, 252–264 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber J. P., et al., ICOS maintains the T follicular helper cell phenotype by down-regulating Krüppel-like factor 2. J. Exp. Med. 212, 217–233 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warnatz K., et al., Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood 107, 3045–3052 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Roussel L., et al., Loss of human ICOSL results in combined immunodeficiency. J. Exp. Med. 215, 3151–3164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansaldo E., et al., Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 364, 1179–1184 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch M. A., et al., Maternal IgG and IgA antibodies dampen mucosal T helper cell responses in early life. Cell 165, 827–841 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunker J. J., et al., Natural polyreactive IgA antibodies coat the intestinal microbiota. Science 358, eaan6619 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fransen F., et al., BALB/c and C57BL/6 mice differ in polyreactive IgA abundance, which impacts the generation of antigen-specific IgA and microbiota diversity. Immunity 43, 527–540 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Palm N. W., et al., Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunker J. J., et al., Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity 43, 541–553 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng M. Y., et al., Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity 44, 647–658 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wijburg O. L., et al., Innate secretory antibodies protect against natural Salmonella typhimurium infection. J. Exp. Med. 203, 21–26 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macpherson A. J., et al., A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222–2226 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Cong Y., Feng T., Fujihashi K., Schoeb T. R., Elson C. O., A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl. Acad. Sci. U.S.A. 106, 19256–19261 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirota K., et al., Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 14, 372–379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamoto S., et al., Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 41, 152–165 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Landuyt A. E., Klocke B. J., Colvin T. B., Schoeb T. R., Maynard C. L., Cutting edge: ICOS-deficient regulatory T cells display normal induction of Il10 but readily downregulate expression of Foxp3. J. Immunol. 202, 1039–1044 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nava G. M., Friedrichsen H. J., Stappenbeck T. S., Spatial organization of intestinal microbiota in the mouse ascending colon. ISME J. 5, 627–638 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van den Abbeele P., et al., Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 7, 949–961 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riva A., et al., A fiber-deprived diet disturbs the fine-scale spatial architecture of the murine colon microbiome. Nat. Commun. 10, 4366 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maynard C. L., et al., Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat. Immunol. 8, 931–941 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Larimore K., Liang L., Bakkour S., Sha W. C., B7h-expressing dendritic cells and plasma B cells mediate distinct outcomes of ICOS costimulation in T cell-dependent antibody responses. BMC Immunol. 13, 29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gigliotti C. L., et al., ICOS-ligand triggering impairs osteoclast differentiation and function in vitro and in vivo. J. Immunol. 197, 3905–3916 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Shouval D. S., et al., Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 40, 706–719 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kubinak J. L., et al., MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe 17, 153–163 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro-Dopico T., Clatworthy M. R., IgG and Fcγ receptors in intestinal immunity and inflammation. Front. Immunol. 10, 805 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton A. L., et al., Serologic antibodies in relation to outcome in postoperative Crohn’s disease. J. Gastroenterol. Hepatol. 32, 1195–1203 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Lodes M. J., et al., Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Invest. 113, 1296–1306 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duck L. W., et al., Isolation of flagellated bacteria implicated in Crohn’s disease. Inflamm. Bowel Dis. 13, 1191–1201 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Christmann B. S., et al., Human seroreactivity to gut microbiota antigens. J. Allergy Clin. Immunol. 136, 1378–1386.e1-5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burmeister Y., et al., ICOS controls the pool size of effector-memory and regulatory T cells. J. Immunol. 180, 774–782 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Guo F., Iclozan C., Suh W. K., Anasetti C., Yu X. Z., CD28 controls differentiation of regulatory T cells from naive CD4 T cells. J. Immunol. 181, 2285–2291 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kornete M., Sgouroudis E., Piccirillo C. A., ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J. Immunol. 188, 1064–1074 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Redpath S. A., et al., ICOS controls Foxp3(+) regulatory T-cell expansion, maintenance and IL-10 production during helminth infection. Eur. J. Immunol. 43, 705–715 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swidsinski A., et al., Viscosity gradient within the mucus layer determines the mucosal barrier function and the spatial organization of the intestinal microbiota. Inflamm. Bowel Dis. 13, 963–970 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Laidlaw B. J., et al., Interleukin-10 from CD4+ follicular regulatory T cells promotes the germinal center response. Sci. Immunol. 2, eaan4767 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geuking M. B., et al., Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34, 794–806 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W., Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993). [DOI] [PubMed] [Google Scholar]
- 55.Maynard C. L., Weaver C. T., Intestinal effector T cells in health and disease. Immunity 31, 389–400 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elson C. O., et al., Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol. Rev. 206, 260–276 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Kullberg M. C., et al., Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect. Immun. 66, 5157–5166 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L., et al., Natural colonization with Helicobacter species and the development of inflammatory bowel disease in interleukin-10-deficient mice. Helicobacter 10, 223–230 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Xu M., et al., c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554, 373–377 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atarashi K., et al., Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Round J. L., et al., The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeon S. G., et al., Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 8, e1002714 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran H. Q., Ley R. E., Gewirtz A. T., Chassaing B., Flagellin-elicited adaptive immunity suppresses flagellated microbiota and vaccinates against chronic inflammatory diseases. Nat. Commun. 10, 5650 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobayashi K., et al., An FcRn-dependent role for anti-flagellin immunoglobulin G in pathogenesis of colitis in mice. Gastroenterology 137, 1746–1756.e1741 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castro-Dopico T., et al., Anti-commensal IgG drives intestinal inflammation and type 17 immunity in ulcerative colitis. Immunity 50, 1099–1114.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benckert J., et al., The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J. Clin. Invest. 121, 1946–1955 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uo M., et al., Mucosal CXCR4+ IgG plasma cells contribute to the pathogenesis of human ulcerative colitis through FcγR-mediated CD14 macrophage activation. Gut 62, 1734–1744 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Houston S. A., et al., The lymph nodes draining the small intestine and colon are anatomically separate and immunologically distinct. Mucosal Immunol. 9, 468–478 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.