Fig. 4.
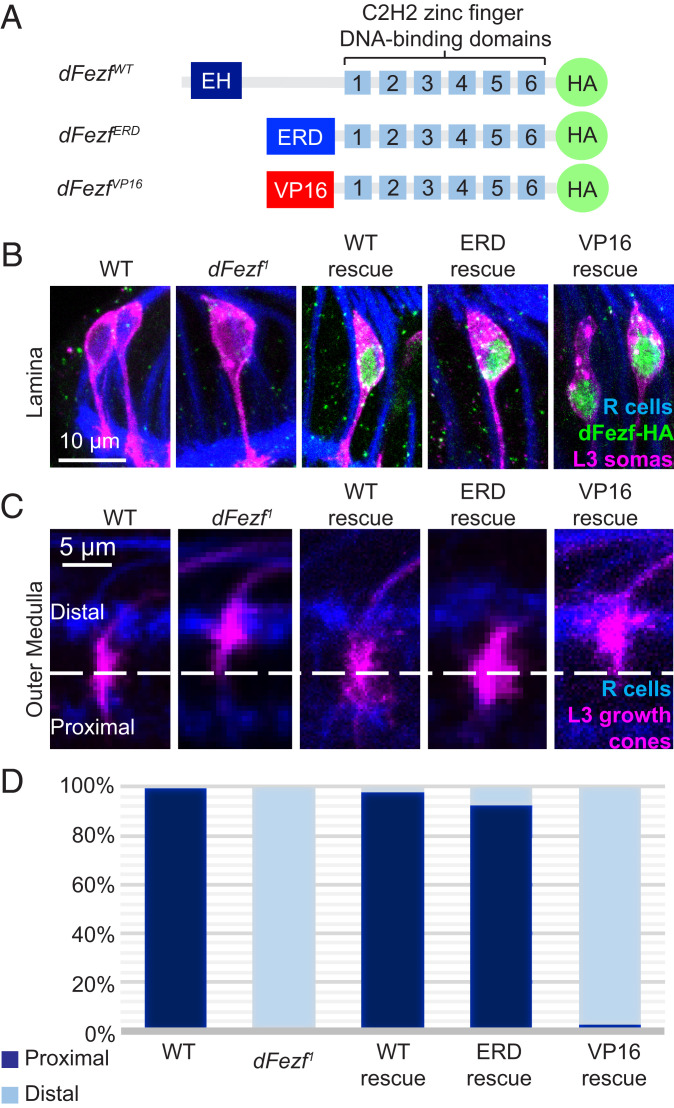
DFezf functions as a transcriptional repressor in regulating L3 broad domain specificity. We performed MARCM rescue experiments using 9-9-GAL4, an L3-specific GAL4 driver, and UAS constructs encoding WT and chimeric forms of dFezf. (A) Schematic illustrating a simplified structure of UAS-dFezf constructs generated in the study by Janssens et al. (21). All three constructs contain six C2H2 zinc finger DNA-binding domains and an HA epitope tag at the C terminus. UAS-dFezfWT contains the WT dFezf structure with an engrailed homology domain (EH) at the N terminus. UAS-dFezfERD contains an ERD at the N terminus. UAS-dFezfVP16 contains a VP16 activation domain (VP16) at the N terminus. (B) Verification of UAS construct expression in MARCM rescue experiments in C. L3 somas are labeled in GFP (magenta). Anti-HA antibody was used to label dFezf-HA (green). R cells are labeled in blue (mAB24B10). Construct expression is confirmed for all three constructs used in this experiment. (C) Confocal images of L3 growth cone targeting at 24 h APF in MARCM rescue experiments using constructs illustrated in A. L3 growth cones are labeled with GFP (magenta). R cells are labeled in blue (mAB24B10). The UAS-dFezfWT and UAS-dFezfERD constructs rescue L3 broad domain specificity (i.e., targeting the proximal domain), while the UAS-dFezfVP16 construct does not. (D) Quantification of data from C.