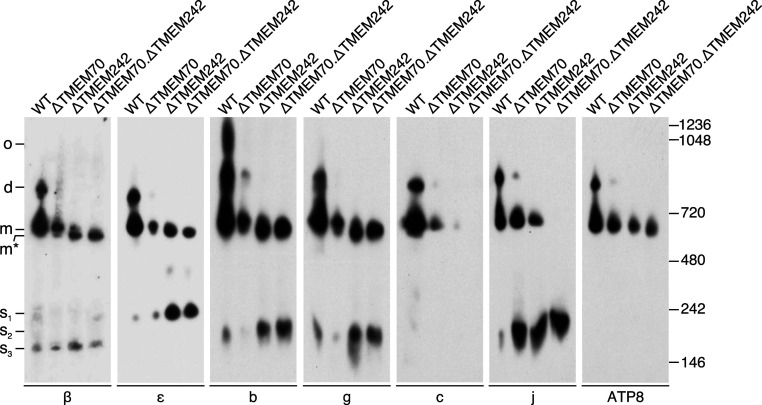
Fig. 5.
Oligomeric states of ATP synthase and vestigial complexes in HAP1 cells devoid of TMEM70 and TMEM242. CN-PAGE analysis of digitonin extracts (detergent:protein, 10:1, g:g) of mitoplasts from HAP1-WT, HAP1-∆TMEM70, HAP1-∆TMEM242, and HAP1-∆TMEM70.∆TMEM242 cells. Below each panel is shown the specificity of the antibody for an individual subunit of ATP synthase employed to detect the complexes related to ATP synthase indicated on the left; d, dimers; m, monomers; m*, the F1-PS-e-f-g complex; o, oligomers; s1, incompletely characterized subcomplex containing the ε-subunit; s2, subcomplex of subunits b, e, f, and g, and of a second subcomplex containing subunit j; s3, incompletely characterized subcomplex containing the β-subunit. The positions of molecular mass markers (kDa) are shown on the right. The total protein in the sample from HAP1-∆TMEM70 cells was twice that from HAP1-WT cells, and those from HAP1-∆TMEM242 and HAP1-∆TMEM70.∆TMEM242 cells were three times greater than HAP1-WT cells. For complementary data, see SI Appendix, Fig. S8.