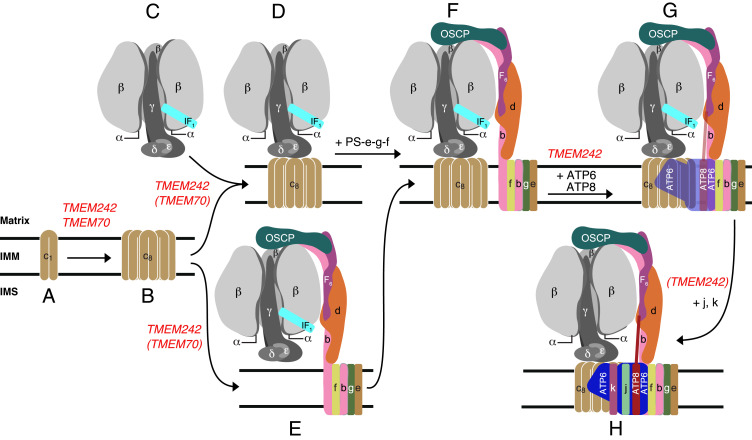
Fig. 8.
Participation of TMEM70 and TMEM242 in the assembly of c8-rings and their incorporation into human ATP synthase. The points where TMEM70 and TMEM242 participate are shown in red, with the parentheses indicating that their participation is suggested by the experimental data, but not proved. (A and B) Eight monomeric c-subunits are assembled into c8-rings. Both TMEM242 and TMEM70 participate in the formation of the membrane bound monomeric c-subunits and in the formation of c8-rings. (C–F) The c8-ring becomes associated with the separately assembled F1-module (C) to form (D) the F1-c8 intermediate (29). In a separate alternate pathway, the c8-ring is incorporated into the F1-PS-e-f-g vestigial complex (E) observed in HAP1-Δc cells (4, 10) to form a key intermediate (F) observed in ρ0 cells (4). TMEM242, and possibly TMEM70 also, participate in the incorporation of the c8-ring into vestigial complexes (D and E). Key intermediate F can be formed also from D by the addition of first, the PS, and then subunits e and g together, and finally subunit f. No assembly factors are known to participate in the conversion of D to F (11). The key intermediate (F) provides the template for the incorporation of the two mitochondrially encoded subunits, ATP6 and ATP8, which is supported by TMEM242 (G). The subsequent addition of subunit j stabilizes their incorporation (10). The addition of subunit k completes the monomeric enzyme (H). The incorporation of both subunits j and k require TMEM242. It is not known when the monomeric complexes associate into the higher oligomeric rows of back-to-face dimers observed along the edges of the cristae. The interface between monomers in back-to-face dimers involves interactions between j-subunits (23). The noncovalent cross-links between monomers in adjacent dimers across the dimer–dimer interface probably involve both g- and k-subunits (41). It is possible these cross-links form from monomeric or dimeric complexes, or both.