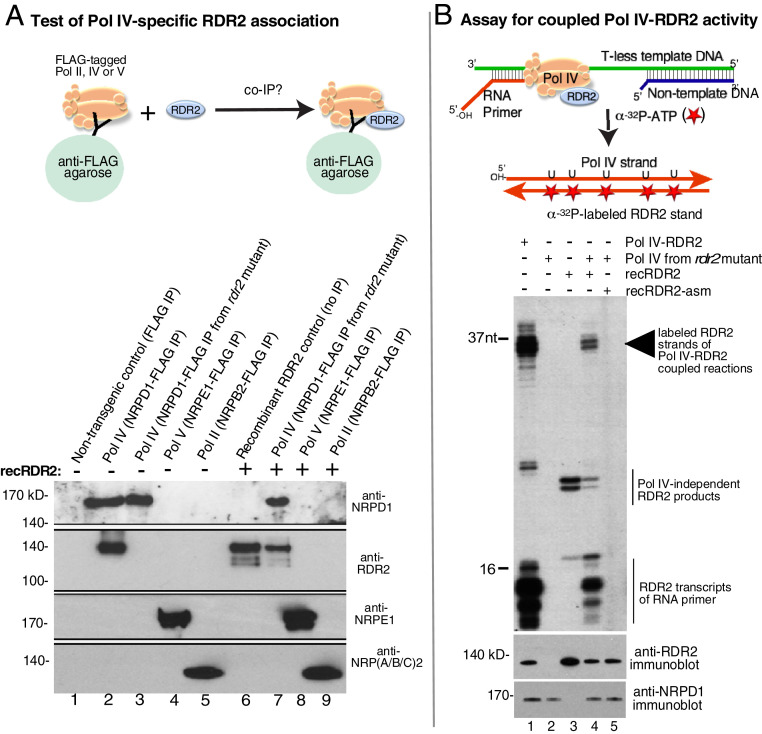
Fig. 2.
Reconstitution of a functional Pol IV-RDR2 complex. (A) Recombinant RDR2 stably associates with Pol IV. Pol IV, Pol V, and Pol II assembled using FLAG-tagged NRPD1, NRPE1, or NRPB2, respectively, were IPed from transgenic plants using anti-FLAG resin. In lane 1, anti-FLAG IP of a nontransgenic plant lysate serves as a negative control. In lane 2, Pol IV was IPed from plants that are wild type for RDR2. In lanes 3 and 7, Pol IV IPed from a rdr2 null mutant. In lanes 7–9, recombinant RDR2 was mixed with the indicated Pol IV, Pol V, or Pol II fractions prior to anti-FLAG IP. Lane 6 shows recombinant RDR2 as a positive control. Proteins resolved by SDS-PAGE were subjected to immunoblotting using anti-RDR2, anti-NRPD1, anti-NRPE1, or an antibody that recognizes a peptide sequence of the Pol II subunit, NRPB2 that is also present in the NRPA2 and NRPC2 subunits of Pols I and III, respectively. (B) Pol IV-RDR2 assembled using recombinant RDR2 carries out the coupled Pol IV-RDR2 reaction, generating dsRNA from a DNA template. The cartoon illustrates the use of a T-less DNA template and RNA labeling with α-[32P]ATP to specifically label second RNA strands synthesized by RDR2 (13). Lane 1 shows the activity of Pol IV-RDR2 complexes isolated from plants expressing WT RDR2. Lane 2 tests the activity of Pol IV purified from the rdr2 null mutant background. Lane 3 tests the activity of recombinant RDR2 alone. In lanes 4 and 5, Pol IV-RDR2 complexes were reconstituted using recombinant RDR2 or catalytically dead RDR2-asm and tested for activity. The bottom image shows immunoblots to detect NRPD1 or RDR2 present in the reactions.