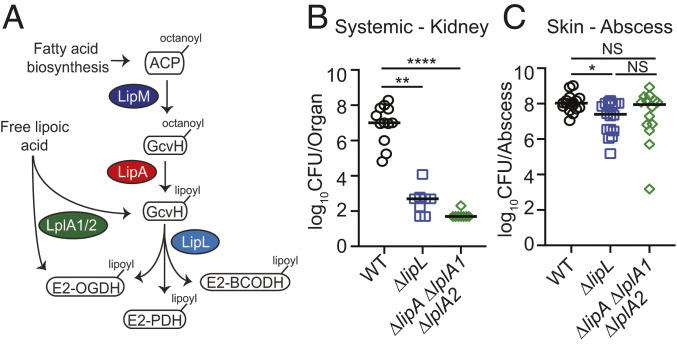
Fig. 1.
Differential requirement for lipoic acid by S. aureus during infection. (A) Lipoic acid synthesis and salvage pathways in S. aureus. De novo synthesis of lipoic acid by S. aureus requires the octanoyltransferase, LipM, and the lipoyl synthase, LipA. The amidotransferase, LipL, shuttles the lipoyl moiety to the E2 subunits of α-ketoacid dehydrogenase complexes that require the cofactor for function. S. aureus also encodes two salvage enzymes, LplA1 and LplA2, to scavenge free lipoic acid from the environment. ACP, acyl carrier protein; GcvH, H subunit glycine cleavage complex; E2-PDH, E2 subunit pyruvate dehydrogenase complex; E2-OGDH, E2 subunit 2-oxoglutarate dehydrogenase complex; E2-BCODH, branched-chain α-ketoacid dehydrogenase complex. (B) Bacterial burden (log10 CFU) in kidneys of mice at 96 h postinfection with 1 × 107 CFU WT (n = 12), ΔlipL (n = 8), and ΔlipA ΔlplA1 ΔlplA2 (n = 8) strains. (C) Bacterial burden (log10 CFU) in skin abscesses of mice at 120 h postinfection with 1 × 107 CFU WT (n = 16), ΔlipL (n = 16), and ΔlipA ΔlplA1 ΔlplA2 (n = 14) strains. P values were determined by a nonparametric one-way ANOVA (Kruskal–Wallis test) with Dunn’s posttest. *P < 0.05, **P < 0.01, ****P < 0.0001, and NS, not significant.