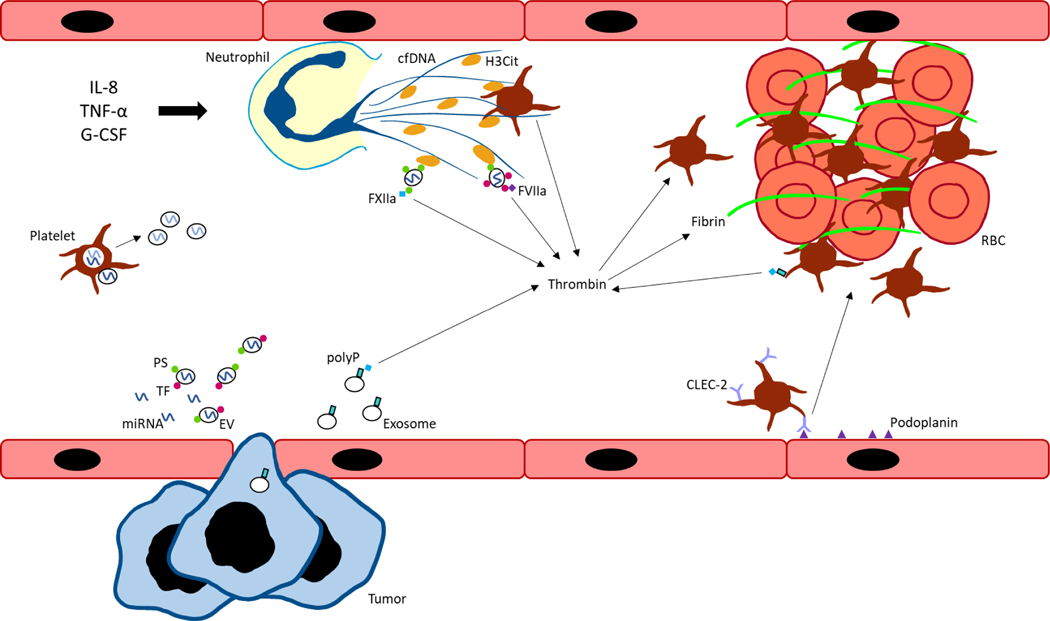
Figure 1. Graphical summary of key mechanisms driving cancer-associated thrombosis.
Interleukin-8 (IL-8), tumor necrosis factor-α (TNF- α), and granulocyte-colony stimulating factor (G-CSF) stimulate neutrophils to form neutrophil extracellular traps (NETs) containing cell free DNA (cfDNA) and citrullinated histone H3 (H3Cit). Extracellular vesicles (EVs), derived from tumor or host cells, express negatively charged phosphatidylserine (PS) which bind histones in NETs. EVs also express tissue factor (TF) which binds factor VIIa (FVIIa) to initiate the extrinsic pathway of coagulation and generate thrombin. Tumor cells can also secrete exosomes expressing polyphosphate (polyP) to activate the contact pathway. Similarly, platelets can express polyP to initiate contact pathway activation, as well as interact with cfDNA and histones present in NETs for thrombin and thrombus formation. Podoplanin-expressing activated endothelial cells can bind C-type lectin-like receptor 2 (CLEC-2) on platelets to induce platelet activation and aggregation. Inhibitory microRNAs (miRNAs) can be secreted extracellularly in a protein- or EV-bound form. Platelets and other host cells can take up miRNAs which regulate gene expression of coagulation factors and re-secrete miRNAs into plasma. FXIIa, factor XIIa; RBC, red blood cell.